Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage - PubMed (original) (raw)
Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage
Nicoletta Kessaris et al. Nat Neurosci. 2006 Feb.
Abstract
The developmental origin of oligodendrocyte progenitors (OLPs) in the forebrain has been controversial. We now show, by Cre-lox fate mapping in transgenic mice, that the first OLPs originate in the medial ganglionic eminence (MGE) and anterior entopeduncular area (AEP) in the ventral forebrain. From there, they populate the entire embryonic telencephalon including the cerebral cortex before being joined by a second wave of OLPs from the lateral and/or caudal ganglionic eminences (LGE and CGE). Finally, a third wave arises within the postnatal cortex. When any one population is destroyed at source by the targeted expression of diphtheria toxin, the remaining cells take over and the mice survive and behave normally, with a normal complement of oligodendrocytes and myelin. Thus, functionally redundant populations of OLPs compete for space in the developing brain. Notably, the embryonic MGE- and AEP-derived population is eliminated during postnatal life, raising questions about the nature and purpose of the competition.
Conflict of interest statement
Competing Interests statement
The authors declare that they have no competing financial interests.
Figures
Figure 1
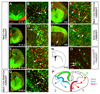
Three successive waves of OLs generated from distinct precursor populations at different times during forebrain development. (a, b) Generation of the first wave of Pdgfra+ OLPs begins at E12.5 from_Nkx2.1_-expressing precursors in the MGE/AEP. These OLPs express GFP (green) in Nkx2.1-Cre/ Rosa26R-GFP embryos (arrows inb). (c, d) By E14.5 Pdgfra+, GFP+ cells start to appear at the cortico-striatal boundary in Nkx2.1-Cre/ Rosa26R-GFP embryos (arrows in d), demonstrating migration of MGE/AEP-derived OLPs into the developing cortex. (e, f) By E16.5 a new wave of Pdgfra+, GFP-negative OLPs is observed in_Nkx2.1-Cre/ R26R-GFP_ cortex; these must be generated from_Nkx2.1_-negative precursors (arrowheads in f). (g, h) All Pdgfra+ cells in the telencephalon co-express GFP in_Nkx2.1-Cre/ Gsh2-Cre/ Rosa26R-GFP_ triple-transgenic embryos (arrows in h), indicating that all OLPs are derived from the ventral forebrain (MGE/AEP + LGE/CGE) at this stage. (i, j) Another wave of Pdgfra+, GFP-negative OLPs appears in the telencephalon of early postnatal Nkx2.1-Cre/ Gsh2-Cre/ Rosa26R-GFP mice (arrowheads inj). (k, l) Emx1_-expressing cortical precursors begin to generate OLPs/OLs after birth (arrows inl). (m, n) Tamoxifen administered once to pregnant_Emx1-CreERT2 females at E9.5, before invasion of the cortex by ventrally-derived OLPs, activates Cre recombination in the embryos and results in expression of the GFP reporter in a subset of Sox10+ OLPs (arrows in n)- which must therefore have been generated from endogenous cortical precursors. (o) Conclusion: three sequential waves of OLPs are generated from different parts of the telencephalic VZ: 1) from _Nkx2.1_-expressing precursors starting at E12.5, 2) from_Gsh2_-expressing LGE/CGE precursors starting at E15.5 and 3) from _Emx1_-expressing cortical precursors starting around birth (P0). Scale bars: (a, c, e, g, i, k) 500 μm; (b, d, f, h, j, l, n) 60 μm.
Figure 2
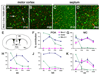
The embryonic _Nkx2.1_-derived OL lineage is rapidly eliminated during postnatal life. (a, b) In Nkx2.1-Cre/ Rosa26R-GFP mice GFP+ (green), Sox10+ (red) double-positive OLPs are already a minority in the P10 cortex (arrows in a), and their contribution drops to zero by P80. (c, d) At P10 practically all OLPs in ventral regions such as the septum are derived from_Nkx2.1_-expressing (MGE/AEP-derived) precursors (GFP, Sox10 double-positive; yellow nuclei in c) but even here they are replaced by other populations (GFP-negative) by P80. (e-j) The proportional contributions of the three different populations of Sox10+ OL lineage cells are presented as percentage of the total number of Sox10+ cells in each region (average± standard deviation). OLs/OLPs derived from_Nkx2.1_-expressing precursors are largely eliminated between birth and adulthood from most regions examined. OLs/OLPs generated from cortical precursors (_Emx1_-derived) remain within the cortex at all stages. _Gsh2_-expressing precursors give rise to OLs/OLPs that spread throughout the telencephalon. POA, preoptic area; MC, motor cortex; AC, anterior commissure; LOT, lateral olfactory tract; CC, corpus callosum. Scale bar: 100 μm.
Figure 3
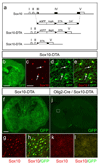
Design and activity of a Cre-inducible Diphtheria toxin (DTA) transgene under_Sox10_ transcriptional control. (a) Intron/exon structure of the Sox10 locus (top). The genomic region spanning exons III – V was replaced with the cassette_lox-eGFP-polyA4-lox-DTA-frt-Cmr-frt_by homologous recombination in bacteria. The Cmr cassette was then removed by transient activation of Flp recombinase in bacteria, producing a latent DTA transgene that expresses GFP under_Sox10_ transcriptional control (Sox10-DTA). In the presence of Cre recombinase the GFP-polyA cassette is removed, thus activating the DTA cassette and killing the expressing cells (further details on request). (b-e) Coronal section through the telencephalon of a Sox10-DTA transgenic mouse at P3 and high magnification images of the cortex from the same section. All Sox10+ OLPs/OLs in the Sox10-DTA mouse co-express GFP, demonstrating the veracity of transgene expression. (f-k) The DTA transgene was activated by crossing Sox10-DTA to an_Olig2-Cre_ mouse, excising the GFP cassette in all Olig2-expressing precursors and their descendants, hence permitting expression of DTA and highly specific killing of all Sox10+ OL lineage cells. The effectiveness of this tool is demonstrated by the loss of all (Sox10+, GFP+) OL lineage cells in the double-transgenics (compare f-h withi-k). The Olig2-Cre/Sox10-DTA mice died shortly after birth, apparently from a motor deficit that prevented them from feeding. Scale bars: (b) 1 mm; (c-e) 50 μm; (f, i) 500 μm; (g-h, j-k) 80 μm.
Figure 4
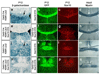
Genetic ablation of region-specific OL populations reveals functional redundancy and compensation among the different lineages. (a-d)_Gsh2_-derived white matter glia (mainly OL lineage cells) were visualized in the corpus callosum (a-b) or anterior commissure (c-d) of P12 Gsh2-Cre/Rosa26R-lacZ(a,c) and Gsh2-Cre/ Sox10-DTA/ Rosa26-R-lacZ(b,d) mice (coronal sections, arrows). Many blue cells are visible in these tracts in Gsh2-Cre/Rosa26R-lacZ mice but are missing from the equivalent structures of Gsh2-Cre/ Sox10-DTA/ Rosa26-R-lacZ mice, showing that _Gsh2_-derived OLs have been effectively ablated in the latter through the action of DTA. (e-l) Despite the specific loss of_Gsh2_-derived OLs, there is no apparent reduction in the total complement of OLs in these white matter tracts as visualized by GFP staining (compare e with f, g with h) or Sox10 staining (compare i with j and kwith l). (m-p) There is no apparent reduction in the amount of myelin in adult white matter tracts of ablated Gsh2-Cre/ Sox10-DTA mice as visualized by Sudan black histochemistry (comparem with n, o with p). Thus it appears that the loss of _Gsh2_-derived OLs is compensated for by the invasion of alternative OL lineages. Similar compensation was observed when the _Emx1-derived OL lineage was ablated in_Emx1-Cre/ Sox10-DTA mice (data available on request). The mice resulting from these crosses survived, behaved and reproduced normally until at least P60. These experiments demonstrate that the different regional OL lineages compete for territory in the normal developing CNS and further suggest that the different OL populations are functionally equivalent. Scale bar: 400 μm.
Figure 5
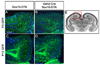
Transient delay in accumulation of OLPs after ablation of the LGE/CGE-derived population in Gsh2-Cre/Sox10-DTA mice. Coronal forebrain sections of P3 or P15 mice were immunolabeled for GFP (green) and Sox10 (not shown). GFP+ cells are Sox10-expressing OLPs that have NOT undergone Cre recombination to excise GFP and activate DTA. (a, b) Posterior, dorso-medial cortex of non-ablated mice. GFP+ cells are more-or-less evenly distributed through the outer cortex of control mice, both at P3 and P15. (c, d) Ablated mice. Accumulation of OLPs is delayed in the outer cortex following ablation of the LGE/CGE-derived population (comparea, c). The distribution is normalized by P15 (compare b, d). Since LGE/CGE-derived OLPs are absent from the ablated animals (c, d), neighbouring populations must make up the difference. For orientation of sections see (e). Scale bars: 300 μm.
Comment in
- Telencephalic oligodendrocytes battle it out.
Ventura RE, Goldman JE. Ventura RE, et al. Nat Neurosci. 2006 Feb;9(2):153-4. doi: 10.1038/nn0206-153. Nat Neurosci. 2006. PMID: 16439977 No abstract available.
Similar articles
- Embryonic origins of forebrain oligodendrocytes revisited by combinatorial genetic fate mapping.
Cai Y, Zhao Z, Shi M, Zheng M, Gong L, He M. Cai Y, et al. Elife. 2024 Sep 11;13:RP95406. doi: 10.7554/eLife.95406. Elife. 2024. PMID: 39259216 Free PMC article. - Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon.
Tekki-Kessaris N, Woodruff R, Hall AC, Gaffield W, Kimura S, Stiles CD, Rowitch DH, Richardson WD. Tekki-Kessaris N, et al. Development. 2001 Jul;128(13):2545-54. doi: 10.1242/dev.128.13.2545. Development. 2001. PMID: 11493571 - Spatiotemporally different origins of NG2 progenitors produce cortical interneurons versus glia in the mammalian forebrain.
Tsoa RW, Coskun V, Ho CK, de Vellis J, Sun YE. Tsoa RW, et al. Proc Natl Acad Sci U S A. 2014 May 20;111(20):7444-9. doi: 10.1073/pnas.1400422111. Epub 2014 May 5. Proc Natl Acad Sci U S A. 2014. PMID: 24799701 Free PMC article. - Transcriptional Regulation of Cortical Interneuron Development.
Pai ELL, Vogt D, Hu JS, Rubenstein JL. Pai ELL, et al. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 47. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 47. PMID: 39637134 Free Books & Documents. Review. - Oligodendrocyte lineage and the motor neuron connection.
Richardson WD, Smith HK, Sun T, Pringle NP, Hall A, Woodruff R. Richardson WD, et al. Glia. 2000 Jan 15;29(2):136-42. doi: 10.1002/(sici)1098-1136(20000115)29:2<136::aid-glia6>3.0.co;2-g. Glia. 2000. PMID: 10625331 Review.
Cited by
- Regulation of miRNA 219 and miRNA Clusters 338 and 17-92 in Oligodendrocytes.
de Faria O Jr, Cui QL, Bin JM, Bull SJ, Kennedy TE, Bar-Or A, Antel JP, Colman DR, Dhaunchak AS. de Faria O Jr, et al. Front Genet. 2012 Mar 28;3:46. doi: 10.3389/fgene.2012.00046. eCollection 2012. Front Genet. 2012. PMID: 22470405 Free PMC article. - Shaping our minds: stem and progenitor cell diversity in the mammalian neocortex.
Franco SJ, Müller U. Franco SJ, et al. Neuron. 2013 Jan 9;77(1):19-34. doi: 10.1016/j.neuron.2012.12.022. Neuron. 2013. PMID: 23312513 Free PMC article. Review. - Calcium and activity-dependent signaling in the developing cerebral cortex.
Arjun McKinney A, Petrova R, Panagiotakos G. Arjun McKinney A, et al. Development. 2022 Sep 1;149(17):dev198853. doi: 10.1242/dev.198853. Epub 2022 Sep 14. Development. 2022. PMID: 36102617 Free PMC article. Review. - miR-200 family controls late steps of postnatal forebrain neurogenesis via Zeb2 inhibition.
Beclin C, Follert P, Stappers E, Barral S, Coré N, de Chevigny A, Magnone V, Lebrigand K, Bissels U, Huylebroeck D, Bosio A, Barbry P, Seuntjens E, Cremer H. Beclin C, et al. Sci Rep. 2016 Oct 21;6:35729. doi: 10.1038/srep35729. Sci Rep. 2016. PMID: 27767083 Free PMC article. - Progenies of NG2 glia: what do we learn from transgenic mouse models ?
Guo Q, Scheller A, Huang W. Guo Q, et al. Neural Regen Res. 2021 Jan;16(1):43-48. doi: 10.4103/1673-5374.286950. Neural Regen Res. 2021. PMID: 32788446 Free PMC article.
References
- Sun T, Pringle NP, Hardy AP, Richardson WD, Smith HK. Pax6 influences the time and site of origin of glial precursors in the ventral neural tube. Mol Cell Neurosci. 1998;12:228–239. - PubMed
- Lu QR, et al. Common developmental requirement for oligodendrocyte lineage gene (Olig) function indicates a motor neuron/oligodendrocyte lineage connection. Cell. 2002;109:75–86. - PubMed
- Takebayashi H, et al. The basic helix-loop-helix factor Olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol. 2002;12:1157–1163. - PubMed
- Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. - PubMed
- Cai J, et al. Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron. 2005;45:41–53. - PubMed
Publication types
MeSH terms
Grants and funding
- 066745/WT_/Wellcome Trust/United Kingdom
- G0501173/MRC_/Medical Research Council/United Kingdom
- G0800575/MRC_/Medical Research Council/United Kingdom
- G9708005/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases