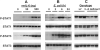Roles of interleukin-6 in activation of STAT proteins and recruitment of neutrophils during Escherichia coli pneumonia - PubMed (original) (raw)
. 2006 Feb 1;193(3):360-9.
doi: 10.1086/499312. Epub 2005 Dec 27.
Affiliations
- PMID: 16388483
- PMCID: PMC2674298
- DOI: 10.1086/499312
Roles of interleukin-6 in activation of STAT proteins and recruitment of neutrophils during Escherichia coli pneumonia
Matthew R Jones et al. J Infect Dis. 2006.
Abstract
Interleukin (IL)-6 concentrations are positively associated with the severity of pneumonia, and this cytokine is essential to surviving experimental pneumococcal pneumonia. The role that IL-6 plays during pneumonia and its impact during gram-negative bacterial pneumonia remain to be determined. During Escherichia coli pneumonia, IL-6-deficient mice had increased bacterial burdens in their lungs, indicating compromised host defenses. Decreased neutrophil counts in alveolar air spaces, despite normal blood neutrophil counts and survival of emigrated neutrophils, suggested that defective neutrophil recruitment was responsible for exacerbating the infection. Neutrophil recruitment requires nuclear factor (NF)- kappa B, but IL-6 was neither sufficient nor essential to induce NF- kappa B-mediated gene expression in the lungs. In contrast, IL-6 induced the phosphorylation of signal transducer and activator of transcription (STAT) 1 and STAT3 in the lungs, and STAT1 and STAT3 phosphorylation during E. coli pneumonia was decreased by IL-6 deficiency. Thus, IL-6 plays essential roles in activating STAT transcription factors, enhancing neutrophil recruitment, and decreasing bacterial burdens during E. coli pneumonia.
Figures
Figure 1
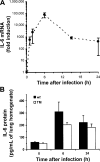
Interleukin (IL)–6 in the lungs during Escherichia coli pneumonia. A, IL-6 mRNA in the lungs. IL-6 mRNA increased within 1 h of infection and remained increased through 24 h after infection. IL-6 and _β_-actin mRNA were measured in lung homogenates using quantitative reverse-transcription polymerase chain reaction. IL-6 transcripts were normalized to β_-actin transcripts for each sample. The effect of time on IL-6 mRNA was statistically significant (P<.05, 1-way analysis of variance [ANOVA]). B, IL-6 protein in the lungs. IL-6 protein increased during infection of either wild-type mice (n = 5 mice/group) or triple mutant (TM) mice (n = 5 mice in the 0-h group, n = 4 mice in the 6-h group, and n = 6 mice in the 24-h group) lacking signaling receptors for tumor necrosis factor–_α and IL-1. IL-6 concentrations in lung homogenates were determined using ELISA. There was a statistically significant effect of time (P<.05, 2-way ANOVA) but not of genotype.
Figure 2
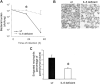
Effects of interleukin (IL)–6 deficiency on neutrophil accumulation and bacterial clearance during Escherichia coli pneumonia. A, Bacterial clearance and IL-6 deficiency. Living bacteria were quantified in lung homogenates collected from wild-type (wt) and IL-6–deficient mice 24 h after infection (n = 5−9 mice/group). The effect of time on colony-forming units was statistically significant (P<.05 , 2-way analysis of variance [ANOVA]), and so was the effect of genotype (denoted by asterisk; P<.05, 2-way ANOVA). B, Effect of IL-6 deficiency on accumulation of neutrophils in alveolar air spaces. Representative images are shown of pulmonary parenchyma from lungs of wt and IL-6–deficient mice. Left lung lobes were collected 24 h after infection, fixed by the instillation of 6% glutaraldehyde at 23 cm H2O pressure, and embedded in paraffin, and 5-mm sections were stained with hematoxylin-eosin. Histological analyses revealed acute inflammation with a diffuse and patchy distribution throughout the left lung lobe parenchyma. Polymerized proteinaceous material was prominent in alveolar air spaces. Emigrated neutrophils were present in alveolar air spaces, with more apparent in lungs from wt mice than in those from IL-6–deficient mice. C, Emigrated neutrophils in alveolar air spaces of IL-6–deficient mice. Emigrated neutrophils in lung sections (n = 7 mice/group) 24 h after infection, as in panel B, were quantified using standard point-counting morphometric techniques. The relative volumes of the parenchymal regions occupied by emigrated neutrophils were calculated by investigators blinded to the identities of the mice and were expressed as a percentage of the total parenchymal region volume (including both tissue and air spaces). *P<.05, Student's t test.
Figure 3
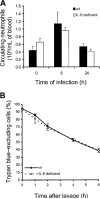
Effect of interleukin (IL)–6 deficiency on blood neutrophils and survival of emigrated neutrophils. A, Neutrophils in venous blood from wild-type (wt) and IL-6–deficient mice with Escherichia coli pneumonia. Neutrophils were quantified in venous blood samples from mice 0, 6, or 24 h after infection. There was a significant effect of time (P<.05, 2-way analysis of variance [ANOVA]) but not of genotype on blood neutrophil counts. B, IL-6 deficiency and the life span of emigrated neutrophils. Emigrated neutrophils were collected from alveolar air spaces of wt and IL-6–deficient mice by bronchoalveolar lavage 24 h after infection and were cultured in vitro for the designated time. Viability was assessed using trypan blue exclusion, with neutrophils collected from 4 mice/group. The viability of emigrated neutrophils decreased over time but did not differ between genotypes (2-way ANOVA).
Figure 4
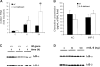
Interleukin (IL)–6 and NF-_κ_B–mediated gene expression during Escherichia coli pneumonia. A, Intercellular adhesion molecule (ICAM)–1 RNA concentrations. Steady-state concentrations of ICAM-1 RNA did not differ between wild-type (wt) and IL-6–deficient mice. Lungs were collected from 10 mice/genotype at 6 h or from 3 mice/genotype at 0 and 24 h after infection. ICAM-1 transcripts were measured by quantitative reverse-transcription polymerase chain reaction, normalized to 18s rRNA, and then expressed as the fold induction from 0-h levels, which did not differ between genotypes. Both time and genotype had significant effects (denoted by asterisk; P<.05 , 2-way analysis of variance and Bonferroni post hoc test). B, Concentrations of the NF-_κ_B–dependent chemokines KC and macrophage inflammatory protein (MIP)–2. Chemokine concentrations did not differ between wt and IL-6–deficient mice (n = 5−6 mice/group). Lungs were collected 6 h after infection, and chemokine concentrations in bronchoalveolar lavage fluid (BAL) were determined using ELISA. There was no significant effect of genotype (Student's t test). C, Inhibitor of NF-_κ_B (I_κ_B) content in the lungs. I_κ_B content decreased during E. coli pneumonia but did not differ between genotypes. The I_κ_B content was assessed by immunoblotting at the indicated time after infection. Each lane contains protein from an individual mouse that was either homozygous (+/+) or mutant (−/−) for the Il6 gene. D, I_κ_B content in the lungs and IL-6. I_κ_B content was not affected by the intratracheal instillation of recombinant murine (rm) IL-6. I_κ_B content in wt mice that received the indicated dose of rmIL-6 was assessed by immunoblotting. Each lane contains protein from an individual mouse.
Figure 5
Interleukin (IL)–6 and activation of signal transducer and activator of transcription (STAT) proteins in the lungs during Escherichia coli pneumonia. Concentrations of tyrosine-phosphorylated (P) and total STAT proteins in the lungs were assessed by immunoblotting. Each lane contains protein from an individual mouse. A, IL-6 and phosphorylation of STAT1 and STAT3 in the lungs. Recombinant murine (rm) IL-6 was instilled intratracheally to wild-type (wt) mice at the indicated dose per mouse, and lungs were collected 1 h later. The instillation of rmIL-6 was sufficient to induce phosphorylation. B, Phosphorylation of STAT1 and STAT3 during E. coli pneumonia. Lungs were collected from wt mice at the indicated time after infection, and phosphorylation was assessed. C, IL-6 deficiency and STAT1 and STAT3 phosphorylation. Lungs were collected from wt and IL-6–deficient mice 6 h after infection, and phosphorylation was assessed. IL-6 deficiency decreased phosphorylation.
Similar articles
- Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia.
Quinton LJ, Jones MR, Robson BE, Simms BT, Whitsett JA, Mizgerd JP. Quinton LJ, et al. Am J Respir Cell Mol Biol. 2008 Jun;38(6):699-706. doi: 10.1165/rcmb.2007-0365OC. Epub 2008 Jan 10. Am J Respir Cell Mol Biol. 2008. PMID: 18192501 Free PMC article. - Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF)-mediated signaling contributes to innate immune responses in the lung during Escherichia coli pneumonia.
Jeyaseelan S, Young SK, Fessler MB, Liu Y, Malcolm KC, Yamamoto M, Akira S, Worthen GS. Jeyaseelan S, et al. J Immunol. 2007 Mar 1;178(5):3153-60. doi: 10.4049/jimmunol.178.5.3153. J Immunol. 2007. PMID: 17312163 - CD44 deficiency leads to enhanced neutrophil migration and lung injury in Escherichia coli pneumonia in mice.
Wang Q, Teder P, Judd NP, Noble PW, Doerschuk CM. Wang Q, et al. Am J Pathol. 2002 Dec;161(6):2219-28. doi: 10.1016/S0002-9440(10)64498-7. Am J Pathol. 2002. PMID: 12466136 Free PMC article. - Early response cytokines and innate immunity: essential roles for TNF receptor 1 and type I IL-1 receptor during Escherichia coli pneumonia in mice.
Mizgerd JP, Spieker MR, Doerschuk CM. Mizgerd JP, et al. J Immunol. 2001 Mar 15;166(6):4042-8. doi: 10.4049/jimmunol.166.6.4042. J Immunol. 2001. PMID: 11238652 - Neutrophil recruitment to the lungs during bacterial pneumonia.
Craig A, Mai J, Cai S, Jeyaseelan S. Craig A, et al. Infect Immun. 2009 Feb;77(2):568-75. doi: 10.1128/IAI.00832-08. Epub 2008 Nov 17. Infect Immun. 2009. PMID: 19015252 Free PMC article. Review. No abstract available.
Cited by
- The known and missing links between Toxoplasma gondii and schizophrenia.
Elsheikha HM, Büsselberg D, Zhu XQ. Elsheikha HM, et al. Metab Brain Dis. 2016 Aug;31(4):749-59. doi: 10.1007/s11011-016-9822-1. Epub 2016 Apr 4. Metab Brain Dis. 2016. PMID: 27041387 Review. - Activation of Hepatic STAT3 Maintains Pulmonary Defense during Endotoxemia.
Hilliard KL, Allen E, Traber KE, Kim Y, Wasserman GA, Jones MR, Mizgerd JP, Quinton LJ. Hilliard KL, et al. Infect Immun. 2015 Oct;83(10):4015-27. doi: 10.1128/IAI.00464-15. Epub 2015 Jul 27. Infect Immun. 2015. PMID: 26216424 Free PMC article. - Roles of STAT3 in protein secretion pathways during the acute-phase response.
Ahyi AN, Quinton LJ, Jones MR, Ferrari JD, Pepper-Cunningham ZA, Mella JR, Remick DG, Mizgerd JP. Ahyi AN, et al. Infect Immun. 2013 May;81(5):1644-53. doi: 10.1128/IAI.01332-12. Epub 2013 Mar 4. Infect Immun. 2013. PMID: 23460517 Free PMC article. - Roles of interleukin-11 during acute bacterial pneumonia.
Traber KE, Dimbo EL, Symer EM, Korkmaz FT, Jones MR, Mizgerd JP, Quinton LJ. Traber KE, et al. PLoS One. 2019 Aug 15;14(8):e0221029. doi: 10.1371/journal.pone.0221029. eCollection 2019. PLoS One. 2019. PMID: 31415618 Free PMC article. - Integrative Physiology of Pneumonia.
Quinton LJ, Walkey AJ, Mizgerd JP. Quinton LJ, et al. Physiol Rev. 2018 Jul 1;98(3):1417-1464. doi: 10.1152/physrev.00032.2017. Physiol Rev. 2018. PMID: 29767563 Free PMC article. Review.
References
- Michaud CM, Murray CJL, Bloom BR. Burden of disease—implications for future research. JAMA. 2001;285:535–9. - PubMed
- Simonsen L, Conn LA, Pinner RW, Teutsch S. Trends in infectious disease hospitalizations in the United States, 1980−1994. Arch Intern Med. 1998;158:1923–8. - PubMed
- Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. JAMA. 1999;281:61–6. - PubMed
- Marrie TJ, Fine MJ, Obrosky DS, Coley C, Singer DE, Kapoor WN. Community-acquired pneumonia due to Escherichia coli. Clin Microbiol Infect. 1998;4:717–23. - PubMed
- Ruiz M, Ewig S, Marcos MA, et al. Etiology of community-acquired pneumonia: impact of age, comorbidity, and severity. Am J Respir Crit Care Med. 1999;160:397–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL079392-02/HL/NHLBI NIH HHS/United States
- HL-07118/HL/NHLBI NIH HHS/United States
- HL-68153/HL/NHLBI NIH HHS/United States
- R01 HL068153-04/HL/NHLBI NIH HHS/United States
- P30 ES000002/ES/NIEHS NIH HHS/United States
- T32 HL007118/HL/NHLBI NIH HHS/United States
- R01 HL068153/HL/NHLBI NIH HHS/United States
- ES-00002/ES/NIEHS NIH HHS/United States
- R01 HL079392/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous