Involvement of the Gi/o/cGMP/PKG pathway in the AT2-mediated inhibition of outer cortex proximal tubule Na+-ATPase by Ang-(1-7) - PubMed (original) (raw)
Involvement of the Gi/o/cGMP/PKG pathway in the AT2-mediated inhibition of outer cortex proximal tubule Na+-ATPase by Ang-(1-7)
Lucienne da Silva Lara et al. Biochem J. 2006.
Abstract
The molecular mechanisms involved in the Ang-(1-7) [angiotensin-(1-7)] effect on sodium renal excretion remain to be determined. In a previous study, we showed that Ang-(1-7) has a biphasic effect on the proximal tubule Na+-ATPase activity, with the stimulatory effect mediated by the AT1 receptor. In the present study, we investigated the molecular mechanisms involved in the inhibition of the Na+-ATPase by Ang-(1-7). All experiments were carried out in the presence of 0.1 nM losartan to block the AT1 receptor-mediated stimulation. In this condition, Ang-(1-7) at 0.1 nM inhibited the Na+-ATPase activity of the proximal tubule by 54%. This effect was reversed by 10 nM PD123319, a specific antagonist of the AT2 receptor, and by 1 muM GDP[beta-S] (guanosine 5'-[beta-thio]diphosphate), an inhibitor of G protein. Ang-(1-7) at 0.1 M induced [35S]GTP[S] (guanosine 5'-[gamma-[35S]thio]triphosphate) binding and 1 mug/ml pertussis toxin, an inhibitor of G(i/o) protein, reversed the Ang-(1-7) effect. Furthermore, it was observed that the inhibitory effect of Ang-(1-7) on the Na+-ATPase activity was completely reversed by 0.1 microM LY83583, an inhibitor of guanylate cyclase, and by 2 muM KT5823, a PKG (protein kinase G) inhibitor, and was mimicked by 10 nM d-cGMP (dibutyryl cGMP). Ang-(1-7) increased the PKG activity by 152% and this effect was abolished by 10 nM PD123319 and 0.1 microM LY83583. Taken together, these data indicate that Ang-(1-7) inhibits the proximal tubule Na+-ATPase by interaction with the AT2 receptor that subsequently activates the G(i/o) protein/cGMP/PKG pathway.
Figures
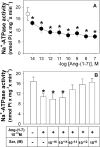
Figure 1. Ang-(1–7) inhibits Na+-ATPase activity
ATPase activity was measured as described in the Experimental section, always in the presence of 0.1 nM losartan. (A) Ang-(1–7) concentrations ranged from 10 fM to 0.1 μM (●). ○, Control in the absence of Ang-(1–7). (B) Effects of saralasin (Sar) at concentrations increasing from 1 fM to 10 nM. Saralasin at 10 nM did not modify the enzyme activity (results not shown). Ang-(1–7) at 0.1 nM was added where indicated (_n_=18). Results are means±S.E.M. *, P<0.05 compared with the control.
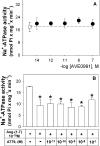
Figure 2. Involvement of the AT1–7 receptor in the modulation of Na+-ATPase activity by Ang-(1–7)
ATPase activity was measured as described in the Experimental section, always in the presence of 0.1 nM losartan. (A) Effects of AVE0991, agonist of the AT1–7 receptor, on enzyme activity. The agonist concentration was increased from 10 fM to 0.1 μM (●). ○, Control in the absence of AVE0991 (_n_=9). (B) Effects of A779, antagonist of the AT1–7 receptor, at concentrations increasing from 10 pM to 0.1 μM. A779 at 10 nM did not modify the enzyme activity (results not shown). Ang-(1–7) at 0.1 nM was added where indicated (_n_=7). Results are means±S.E.M. *, P<0.05 compared with the control.
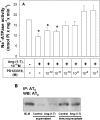
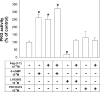
Figure 3. Effects of PD123319, an AT2 receptor antagonist, on the inhibition of Na+-ATPase activity by Ang-(1–7)
(A) ATPase activity was measured as described in the Experimental section, always in the presence of 0.1 nM losartan. The effects of PD123319 concentrations increasing from 1 pM to 0.1 μM are shown. PD123319 at 10 nM did not modify the enzyme activity (results not shown). Ang-(1–7) at 0.1 nM was added where indicated. Results are means±S.E.M. (_n_=8). *, P<0.05 compared with the control. (B) Immunoblot detection of the AT2 receptor in the proximal tubule basolateral membranes. The AT2 receptor was pulled down using an anti-(AT2 receptor) polyclonal antibody. After immunoprecipitation, the basolateral membranes, supernatant and the immunoprecipitated proteins were separated by SDS/10% PAGE, then transferred on to a PVDF membrane and incubated with a primary anti-(AT2 receptor) polyclonal antibody as described in the Experimental section. The blot is representative of three carried out with different preparations. IP, immunoprecipitation; WB, Western blotting; BLM, basolateral membranes without immunoprecipitation.
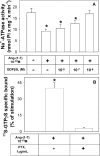
Figure 4. Involvement of G-protein in the inhibition of Na+-ATPase activity by Ang-(1–7)
(A) Effect of GDP[β-S] on Ang-(1–7)-inhibited enzyme activity. ATPase activity was measured as described in the Experimental section, always in the presence of 0.1 nM losartan. GDP[β-S] concentration increased from 10 nM to 1 μM. GDP[β-S] at 1 μM did not modify the enzyme activity (results not shown). Ang-(1–7) at 0.1 nM was added where indicated (_n_=5). (B) Binding of [35S]GTP[S] by basolateral membranes induced by Ang-(1–7). Binding of [35S]GTP[S] was measured as described in the Experimental section, always in the presence of 0.1 nM losartan. Ang-(1–7) at 0.1 nM and 1 μg/ml PTX were added where indicated (_n_=4). Results are means±S.E.M. *, P<0.05 compared with the control.
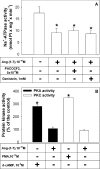
Figure 5. AT2 receptor-mediated Ang-(1–7) effects on Na+-ATPase activity does not involve PLA2, tyrosine kinase, PKC or PKA
(A) Effects of PACOCF3, a plasma membrane-associated PLA2 inhibitor, and genistein, a tyrosine kinase inhibitor, on the inhibition of Na+-ATPase activity by 0.1 nM Ang-(1–7). ATPase activity was measured as described in the Experimental section, always in the presence of 0.1 nM losartan. PACOCF3 and genistein did not modify the enzyme activity (results not shown). The compounds were added where indicated. Results are means±S.E.M. (_n_=4). (B) PKA (closed bars) and PKC (open bars) activities were measured as described in the Experimental section, in the presence of 0.1 nM losartan. Ang-(1–7) at 0.1 nM, d-cAMP at 1 μM or PMA at 1 pM was added where indicated. Results are expressed as percentages of the control (_n_=4). *, P<0.05 compared with the control.
Figure 6. Involvement of the cGMP/PKG pathway in mediating the inhibitory effect of Ang-(1–7) on the Na+-ATPase activity
(A) Effect of LY83583, d-cGMP and KT5823 on the inhibition of the Na+-ATPase activity by 0.1 nM Ang-(1–7). ATPase activity was measured as described in the Experimental section, always in the presence of 0.1 nM losartan. LY83583 at 0.1 μM and KT5823 at 2 μM did not modify the enzyme activity (results not shown). Ang-(1–7) at 0.1 nM, d-cGMP at 10 nM, LY83583 at 0.1 μM or KT5823 at 2 μM were added where indicated. Results are means±S.E.M. (_n_=7). *, P<0.05 compared with the control. (B) Immunoblot detection of PKG in the proximal tubule basolateral membranes. Basolateral membrane proteins were separated by SDS/7.5% PAGE, then transferred on to a PVDF membrane and incubated with a primary anti-PKG monoclonal antibody as described in the Experimental section. The blot is representative of four carried out with different preparations. MW, molecular mass.
Figure 7. Effects of Ang-(1–7) on PKG activity
PKG activity was measured as described in the Experimental section, in the presence of 0.1 nM losartan. Ang-(1–7) at 0.1 nM, d-cGMP at 10 nM, LY83583 at 0.1 μM or PD123319 at 10 nM were added where indicated. Results are expressed as percentages of the control. *, P<0.05 compared with the control (_n_=9).
Similar articles
- Angiotensin II and angiotensin-(1-7) inhibit the inner cortex Na+ -ATPase activity through AT2 receptor.
De Souza AM, Lopes AG, Pizzino CP, Fossari RN, Miguel NC, Cardozo FP, Abi-Abib R, Fernandes MS, Santos DP, Caruso-Neves C. De Souza AM, et al. Regul Pept. 2004 Aug 15;120(1-3):167-75. doi: 10.1016/j.regpep.2004.03.005. Regul Pept. 2004. PMID: 15177935 - Atrial natriuretic peptides and urodilatin modulate proximal tubule Na(+)-ATPase activity through activation of the NPR-A/cGMP/PKG pathway.
Vives D, Farage S, Motta R, Lopes AG, Caruso-Neves C. Vives D, et al. Peptides. 2010 May;31(5):903-8. doi: 10.1016/j.peptides.2010.02.018. Epub 2010 Mar 3. Peptides. 2010. PMID: 20206222 - Angiotensin-(1-7) modulates the ouabain-insensitive Na+-ATPase activity from basolateral membrane of the proximal tubule.
Caruso-Neves C, Lara LS, Rangel LB, Grossi AL, Lopes AG. Caruso-Neves C, et al. Biochim Biophys Acta. 2000 Jul 31;1467(1):189-97. doi: 10.1016/s0005-2736(00)00219-4. Biochim Biophys Acta. 2000. PMID: 10930521 - Dopamine-1 receptor G-protein coupling and the involvement of phospholipase A2 in dopamine-1 receptor mediated cellular signaling mechanisms in the proximal tubules of SHR.
Hussain T, Lokhandwala MF. Hussain T, et al. Clin Exp Hypertens. 1997 Jan-Feb;19(1-2):131-40. doi: 10.3109/10641969709080810. Clin Exp Hypertens. 1997. PMID: 9028641 Review.
Cited by
- Angiotensin-(1-7) inhibits sodium transport via Mas receptor by increasing nitric oxide production in thick ascending limb.
Dibo P, Marañón RO, Chandrashekar K, Mazzuferi F, Silva GB, Juncos LA, Juncos LI. Dibo P, et al. Physiol Rep. 2019 Mar;7(5):e14015. doi: 10.14814/phy2.14015. Physiol Rep. 2019. PMID: 30839176 Free PMC article. - Angiotensin II Type 2 Receptor: A Target for Protection Against Hypertension, Metabolic Dysfunction, and Organ Remodeling.
Fatima N, Patel SN, Hussain T. Fatima N, et al. Hypertension. 2021 Jun;77(6):1845-1856. doi: 10.1161/HYPERTENSIONAHA.120.11941. Epub 2021 Apr 12. Hypertension. 2021. PMID: 33840201 Free PMC article. Review. - The Angiotensin AT2 Receptor: From a Binding Site to a Novel Therapeutic Target.
Steckelings UM, Widdop RE, Sturrock ED, Lubbe L, Hussain T, Kaschina E, Unger T, Hallberg A, Carey RM, Sumners C. Steckelings UM, et al. Pharmacol Rev. 2022 Oct;74(4):1051-1135. doi: 10.1124/pharmrev.120.000281. Pharmacol Rev. 2022. PMID: 36180112 Free PMC article. Review. - Dimerization of AT2 and Mas Receptors in Control of Blood Pressure.
Patel S, Hussain T. Patel S, et al. Curr Hypertens Rep. 2018 May 1;20(5):41. doi: 10.1007/s11906-018-0845-3. Curr Hypertens Rep. 2018. PMID: 29717388 Free PMC article. Review. - Angiotensin-(1-7) protects against the development of aneurysmal subarachnoid hemorrhage in mice.
Shimada K, Furukawa H, Wada K, Wei Y, Tada Y, Kuwabara A, Shikata F, Kanematsu Y, Lawton MT, Kitazato KT, Nagahiro S, Hashimoto T. Shimada K, et al. J Cereb Blood Flow Metab. 2015 Jul;35(7):1163-8. doi: 10.1038/jcbfm.2015.30. Epub 2015 Mar 11. J Cereb Blood Flow Metab. 2015. PMID: 25757758 Free PMC article.
References
- Kucharewicz I., Pawlak R., Matys T., Chabielska E., Buczko W. Angiotensin-(1–7): an active member of the renin–angiotensin system J. Physiol. Pharmacol. 2002;53:533–540. - PubMed
- Handa R. K. Metabolism alters the selectivity of angiotensin-(1–7) receptor ligands for angiotensin receptors. J. Am. Soc. Nephrol. 2000;11:1377–1386. - PubMed
- Santos R. A. S., Campagnole-Santos M. J., Andrade S. P. Angiotensin-(1–7): an update. Regul. Pept. 2000;91:45–62. - PubMed
- Handa R. K., Ferrario C. M., Strandhoy J. W. Renal actions of angiotensin-(1–7): in vivo and in vitro studies. Am. J. Physiol. 1996;270:F141–F147. - PubMed
- Caruso-Neves C., Lara L. S., Rangel L. B., Grossi A. L., Lopes A. G. Angiotensin-(1–7) modulates the ouabain-insensitive Na+-ATPase activity from basolateral membrane of the proximal tubule. Biochim. Biophys. Acta. 2000;1467:189–197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous