Structure of the parainfluenza virus 5 F protein in its metastable, prefusion conformation - PubMed (original) (raw)
Structure of the parainfluenza virus 5 F protein in its metastable, prefusion conformation
Hsien-Sheng Yin et al. Nature. 2006.
Abstract
Enveloped viruses have evolved complex glycoprotein machinery that drives the fusion of viral and cellular membranes, permitting entry of the viral genome into the cell. For the paramyxoviruses, the fusion (F) protein catalyses this membrane merger and entry step, and it has been postulated that the F protein undergoes complex refolding during this process. Here we report the crystal structure of the parainfluenza virus 5 F protein in its prefusion conformation, stabilized by the addition of a carboxy-terminal trimerization domain. The structure of the F protein shows that there are profound conformational differences between the pre- and postfusion states, involving transformations in secondary and tertiary structure. The positions and structural transitions of key parts of the fusion machinery, including the hydrophobic fusion peptide and two helical heptad repeat regions, clarify the mechanism of membrane fusion mediated by the F protein.
Conflict of interest statement
Coordinates and structure factor amplitudes have been deposited in the Protein Data Bank (PDB ID code 2B9B). Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Figures
Figure 1. Structure of SV5 F-GCNt.
a, The F-GCNt domains. Important domains are highlighted in different colours and their corresponding residue ranges are indicated. b, Ribbon diagram of the F trimer, with each chain coloured by residue number in a gradient from blue (N terminus) to red (C terminus). The head and stalk regions are indicated. HRB linker residues (429–432) could not be modelled in one subunit and had high temperature factors in the other two. c, Ribbon diagram of one subunit of the F trimer, coloured by domain. The domains are labelled and the colours correspond to those in a. Arrow indicates the cleavage/activation site. d, Top view of the trimer, coloured as in d. Arrows indicate the cleavage/activation sites. e, Surface representation of the F trimer, coloured by subunit. The fusion peptide exposed surface is shown in blue. f, Close-up view of the fusion peptide (residues 103–128). The peptide is folded back on itself with a small hydrophobic core and contains a mixture of an extended chain, a β-strand and a C-terminal α-helix. The fusion peptide is sandwiched between two subunits of the trimer, between DII and DIII domains.
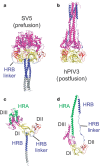
Figure 2. Structural changes between the pre- and postfusion F protein conformations.
a, Ribbon diagram of the SV5 F-GCNt trimer. DI is yellow, DII is red, DIII is magenta, HRB is blue and GCNt is grey. b, Ribbon diagram of the hPIV3 (postfusion) trimer, similarly oriented by DI and coloured as in a. c, Ribbon diagram of a single subunit of the SV5 F-GCNt trimer, coloured as in a except for residues of HRA, which are green. d, Ribbon diagram of a single subunit of the hPIV3 F trimer, coloured as in c.
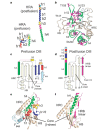
Figure 3. Role of DIII in HRA folding and transformation.
a, HRA refolds from 11 distinct segments (h1, h2, b1, b2, h3, h4 and the intervening residues) in the prefusion conformation into a single helix of ∼120 Å in the postfusion form. b, The HRA helices wrap around the domain III core in the prefusion conformation. The heptad-repeat residues (magenta) do not form any coiled-coil interactions in the prefusion conformation. Breakpoints in the HRA helix (N133, T147, T158 and a stutter observed in the postfusion coiled coil) are labelled. c, Secondary structure of DIII in the prefusion (SV5) conformation. The ‘DIII core’ includes three antiparallel strands, HRC, a helical bundle (HB) and h4 of HRA. Segments of HRA are coloured as in a and the cleavage site (//) and fusion peptide (Fpep) are indicated. The DIII core sheet is extended by the b1 and b2 strands from HRA. d, Secondary structure of DIII in the postfusion (hPIV3) conformation, coloured as in c. The DIII core sheet is extended by one strand from an HRB linker from a neighbouring subunit (magenta). e, Ribbon diagram of DIII in the prefusion conformation, coloured as in c. f, Ribbon diagram of DIII in the postfusion conformation, coloured as in d.
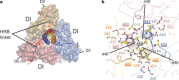
Figure 4. HRB interacts with the base of the head region.
a, View of HRB looking down the three-fold axis from the C terminus of the protein (transmembrane anchor/viral membrane view). The HRB stalk is aligned with the head region along the three-fold axis of the trimer. The HRB linkers extend from the end of DII around the outside of the trimer, before proceeding in towards the top of the HRB stalk, following open grooves in the DI domains. The HRB heptad-repeat residues form the core of the three-helix bundle in the prefusion state, but pack against the HRA coiled coil in the postfusion form. F is coloured by subunit and the HR residues are shown as yellow sticks. b, View of the top of the HRB region looking down the three-fold axis from the base of the head. Interactions between subunits tie together the top of HRB and the base of the head region. The ‘switch peptide’ residues 443, 447 and 449 (yellow) are located here and form intersubunit interactions that explain their role in stabilizing the prefusion conformation.
Figure 5. Model of F-mediated membrane fusion.
a, Structure of the prefusion conformation. HRB is blue, HRA is green, and DI, DII and DIII are yellow, red and magenta, respectively. b, ‘Open stalk’ conformation, in which the HRB stalk melts and separates from the prefusion head region. HRB is shown as three extended chains because the individual segments are unlikely to be helical. This conformation is consistent with a low-temperature intermediate that is inhibited by HRA peptides, but not HRB peptides. Mutations of the switch peptide residues 443, 447 and 449 would influence the formation of this intermediate by affecting stabilizing interactions between the prefusion stalk and head domains (see Fig. 4). c, A pre-hairpin intermediate can form by refolding of DIII, facilitating formation of the HRA coiled coil and insertion of the fusion peptide into the target cell membrane. This intermediate can be inhibited by peptides derived from both HRA and HRB regions. d, Before formation of the final 6HB, folding of the HRB linker onto the newly exposed DIII core, with the formation of additional β-strands (see Fig. 3d, f), may stabilize the juxtaposition of viral and cellular membranes. e, The formation of the postfusion 6HB is tightly linked to membrane fusion and pore formation, juxtaposing the membrane-interacting fusion peptides and transmembrane domains.
Similar articles
- Third Helical Domain of the Nipah Virus Fusion Glycoprotein Modulates both Early and Late Steps in the Membrane Fusion Cascade.
Zamora JLR, Ortega V, Johnston GP, Li J, André NM, Monreal IA, Contreras EM, Whittaker GR, Aguilar HC. Zamora JLR, et al. J Virol. 2020 Sep 15;94(19):e00644-20. doi: 10.1128/JVI.00644-20. Print 2020 Sep 15. J Virol. 2020. PMID: 32669342 Free PMC article. - The Heptad Repeat C Domain of the Respiratory Syncytial Virus Fusion Protein Plays a Key Role in Membrane Fusion.
Bermingham IM, Chappell KJ, Watterson D, Young PR. Bermingham IM, et al. J Virol. 2018 Jan 30;92(4):e01323-17. doi: 10.1128/JVI.01323-17. Print 2018 Feb 15. J Virol. 2018. PMID: 29212939 Free PMC article. - Structure and stabilization of the Hendra virus F glycoprotein in its prefusion form.
Wong JJ, Paterson RG, Lamb RA, Jardetzky TS. Wong JJ, et al. Proc Natl Acad Sci U S A. 2016 Jan 26;113(4):1056-61. doi: 10.1073/pnas.1523303113. Epub 2015 Dec 28. Proc Natl Acad Sci U S A. 2016. PMID: 26712026 Free PMC article. - Structure and function of a paramyxovirus fusion protein.
Morrison TG. Morrison TG. Biochim Biophys Acta. 2003 Jul 11;1614(1):73-84. doi: 10.1016/s0005-2736(03)00164-0. Biochim Biophys Acta. 2003. PMID: 12873767 Review. - Structural basis of viral invasion: lessons from paramyxovirus F.
Lamb RA, Jardetzky TS. Lamb RA, et al. Curr Opin Struct Biol. 2007 Aug;17(4):427-36. doi: 10.1016/j.sbi.2007.08.016. Epub 2007 Sep 17. Curr Opin Struct Biol. 2007. PMID: 17870467 Free PMC article. Review.
Cited by
- Glycan-shielded homodimer structure and dynamical features of the canine distemper virus hemagglutinin relevant for viral entry and efficient vaccination.
Fukuhara H, Yumoto K, Sako M, Kajikawa M, Ose T, Kawamura M, Yoda M, Chen S, Ito Y, Takeda S, Mwaba M, Wang J, Hashiguchi T, Kamishikiryo J, Maita N, Kitatsuji C, Takeda M, Kuroki K, Maenaka K. Fukuhara H, et al. Elife. 2024 Jul 24;12:RP88929. doi: 10.7554/eLife.88929. Elife. 2024. PMID: 39046448 Free PMC article. - Mutagenesis of Paramyxovirus Hemagglutinin-Neuraminidase Membrane-Proximal Stalk Region Influences Stability, Receptor Binding, and Neuraminidase Activity.
Adu-Gyamfi E, Kim LS, Jardetzky TS, Lamb RA. Adu-Gyamfi E, et al. J Virol. 2016 Aug 12;90(17):7778-88. doi: 10.1128/JVI.00896-16. Print 2016 Sep 1. J Virol. 2016. PMID: 27334593 Free PMC article. - Understanding HSV-1 entry glycoproteins.
Reske A, Pollara G, Krummenacher C, Chain BM, Katz DR. Reske A, et al. Rev Med Virol. 2007 May-Jun;17(3):205-15. doi: 10.1002/rmv.531. Rev Med Virol. 2007. PMID: 17295428 Free PMC article. Review. - Virus membrane fusion.
Weissenhorn W, Hinz A, Gaudin Y. Weissenhorn W, et al. FEBS Lett. 2007 May 22;581(11):2150-5. doi: 10.1016/j.febslet.2007.01.093. Epub 2007 Feb 16. FEBS Lett. 2007. PMID: 17320081 Free PMC article. Review. - Side chain packing below the fusion peptide strongly modulates triggering of the Hendra virus F protein.
Smith EC, Dutch RE. Smith EC, et al. J Virol. 2010 Oct;84(20):10928-32. doi: 10.1128/JVI.01108-10. Epub 2010 Aug 11. J Virol. 2010. PMID: 20702638 Free PMC article.
References
- Lamb RA, Kolakofsky D. Fields Virology. 2001. pp. 1305–1340.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources