Ipsilateral hand input to area 3b revealed by converging hemodynamic and electrophysiological analyses in macaque monkeys - PubMed (original) (raw)
Comparative Study
Ipsilateral hand input to area 3b revealed by converging hemodynamic and electrophysiological analyses in macaque monkeys
Michael L Lipton et al. J Neurosci. 2006.
Abstract
Functional magnetic resonance imaging (fMRI) of the hand representation in primary somatosensory cortex (area 3b) of macaque monkeys revealed an ipsilateral hand input undetected by most previous studies. Ipsilateral responses had a hemodynamic signature indistinguishable from that of contralateral hand responses. We explored the neural mechanisms of the fMRI effects using a second derivative analysis of field potentials [current source density (CSD) analysis] combined with action potential profiles, sampled from area 3b using linear array multielectrodes. In contrast to the predominantly excitatory contralateral response, the colocated ipsilateral response appeared dominated by inhibition, suggesting that ipsilateral inputs may have modulatory effects on contralateral input processing. Our findings confirm bimanual convergence at the earliest stage of cortical somatosensory processing in primates. They also illustrate the value of combined CSD and fMRI analyses in monkeys for defining hidden aspects of sensory function and for investigating the neuronal processes generating fMRI signals.
Figures
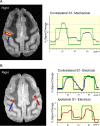
Figure 1.
A, fMRI activation map superimposed on an anatomic image shows robust activation (arrow) localized in the depth of the posterior bank of the central sulcus in the hand region of area 3b contralateral to the left-hand stimulus. Signal intensity time course shows correlation with the stimulus paradigm (green boxcar). B, fMRI activation map obtained during left median nerve stimulation in animal 1 superimposed on an anatomic image showing robust activation precisely localized to the hand region of area 3b contralateral to the side of stimulation (blue arrow) as under the cutaneous stimulus (A). Additionally, activation is evident in the homologous portion of area 3 ipsilateral to the stimulus (maroon arrow). The signal time course demonstrates greater percentage signal change and improved signal-to-noise contralateral to the stimulus (blue) compared with ipsilateral to the stimulus (maroon).
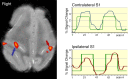
Figure 2.
fMRI activation map obtained during left median nerve stimulation of animal 2 and superimposed on an anatomic image demonstrates reproducible activation of the same regions as in animal 1 both contralateral (blue arrow) and ipsilateral to the stimulus (maroon arrow). The corresponding signal time courses are shown.
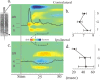
Figure 3.
a, A laminar CSD profile elicited in area 3b by electrical stimulation of the contralateral median nerve (schematic of multielectrode at left). MUA from a contact in layer 4 is superimposed on the CSD profile. Current sinks (red) represent net inward transmembrane current flow in the local neuronal population. Current sources (blue) represent net outward current flow. b, Average onset latency and SE as a function of layer (supragranular, granular, and infragranular) of the response to contralateral median nerve stimulation across all 15 electrode penetrations in three subjects. The values comprising the underlying onset latency distributions were derived by taking the earliest point at which the CSD response deviated by >2 SD units from the baseline and remained so for 8 ms. Mean onsetlatencies were 6.4, 7.3, and 8.0 ms for the granular, supragranular, and infragranular layers, respectively. Latency in layer 4 was significantly earlier (p < 0.05) than in the supragranular or infragranular layers. c, The response in the same site to stimulation of the ipsilateral median nerve. This response has a bilaminar pattern typical of feedback input (responses above and below earlier than in lamina 4), with an apparent inhibition (current source) in lamina 4. MUA decrease (black tracing) is associated with the current source that appears in layer 4 in this condition. d, Mean onset latency and SE as a function of layer from the ipsilateral median nerve-evoked response across the 12 of 15 electrode penetrations showing ipsilateral input in the three subjects. Singular values were derived as described for the contralateral condition. Mean onset latencies for the ipsilateral condition were 44.2, 22.8, and 46.7 ms for granular, supragranular, and infragranular layers, respectively. The supragranular mean latency was significantly earlier (p < 0.05) than the others.
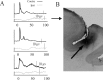
Figure 4.
A, Layer 4 MUA recordings from each of the three monkeys in this study, contrasting the contralateral (Contra) median nerve-evoked response (black traces) to the colocated ipsilateral (Ipsi) response (graytraces). B, Histological reconstruction of a BDA deposition in area 3b, made during one of the last penetrations in subject Y, after the recordings in monkey Y shown at the top of a. BDA was microinjected into layer 4, from a microcannula incorporated into the electrode. BDA localization to area 3b (the posterior bank of the central sulcus above area 3a and below area 1) was confirmed by thick parvalbumin staining of lower layer 3 and layer 4. This helped to verify the location of recording sites within area 3b.
Similar articles
- The organization and connections of anterior and posterior parietal cortex in titi monkeys: do New World monkeys have an area 2?
Padberg J, Disbrow E, Krubitzer L. Padberg J, et al. Cereb Cortex. 2005 Dec;15(12):1938-63. doi: 10.1093/cercor/bhi071. Epub 2005 Mar 9. Cereb Cortex. 2005. PMID: 15758196 - Myelin stains reveal an anatomical framework for the representation of the digits in somatosensory area 3b of macaque monkeys.
Qi HX, Kaas JH. Qi HX, et al. J Comp Neurol. 2004 Sep 13;477(2):172-87. doi: 10.1002/cne.20247. J Comp Neurol. 2004. PMID: 15300788 - Hemispheric asymmetry of hand representation in human primary somatosensory cortex and handedness.
Jung P, Baumgärtner U, Magerl W, Treede RD. Jung P, et al. Clin Neurophysiol. 2008 Nov;119(11):2579-86. doi: 10.1016/j.clinph.2008.04.300. Epub 2008 Sep 10. Clin Neurophysiol. 2008. PMID: 18786858 - Short communication: mapping of somatosensory cortices with functional magnetic resonance imaging in anaesthetized macaque monkeys.
Hayashi T, Konishi S, Hasegawa I, Miyashita Y. Hayashi T, et al. Eur J Neurosci. 1999 Dec;11(12):4451-6. doi: 10.1046/j.1460-9568.1999.00892.x. Eur J Neurosci. 1999. PMID: 10594672 - Task-specific role of ipsilateral pathways: somatosensory evoked potentials during cooperative hand movements.
Schrafl-Altermatt M, Dietz V. Schrafl-Altermatt M, et al. Neuroreport. 2014 Dec 17;25(18):1429-32. doi: 10.1097/WNR.0000000000000285. Neuroreport. 2014. PMID: 25340563
Cited by
- Cortical contributions to sensory gating in the ipsilateral somatosensory cortex during voluntary activity.
Lei Y, Perez MA. Lei Y, et al. J Physiol. 2017 Sep 15;595(18):6203-6217. doi: 10.1113/JP274504. Epub 2017 Aug 18. J Physiol. 2017. PMID: 28513860 Free PMC article. - A Conceptual Model of Tactile Processing across Body Features of Size, Shape, Side, and Spatial Location.
Tamè L, Azañón E, Longo MR. Tamè L, et al. Front Psychol. 2019 Feb 26;10:291. doi: 10.3389/fpsyg.2019.00291. eCollection 2019. Front Psychol. 2019. PMID: 30863333 Free PMC article. Review. - Human orbitofrontal cortex signals decision outcomes to sensory cortex during behavioral adaptations.
Wang BA, Veismann M, Banerjee A, Pleger B. Wang BA, et al. Nat Commun. 2023 Jun 15;14(1):3552. doi: 10.1038/s41467-023-38671-7. Nat Commun. 2023. PMID: 37322004 Free PMC article. - From maps to form to space: touch and the body schema.
Medina J, Coslett HB. Medina J, et al. Neuropsychologia. 2010 Feb;48(3):645-54. doi: 10.1016/j.neuropsychologia.2009.08.017. Epub 2009 Aug 20. Neuropsychologia. 2010. PMID: 19699214 Free PMC article. Review. - Excitatory and inhibitory mechanisms underlying somatosensory habituation.
Klingner CM, Hasler C, Brodoehl S, Witte OW. Klingner CM, et al. Hum Brain Mapp. 2014 Jan;35(1):152-60. doi: 10.1002/hbm.22163. Epub 2012 Jul 30. Hum Brain Mapp. 2014. PMID: 22847930 Free PMC article.
References
- Backes WH, Mess WH, van Kranen-Mastenbroek V, Reulen JP (2000) Somotosensory cortex responses to median nerve stimulation: fMRI effects of current amplitude and selective attention. Clin Neurophysiol 111: 1738–1744. - PubMed
- Barna JS, Arezzo JC, Vaughan Jr HG (1981) A new multielectrode array for the simultaneous recording of field potentials and unit activity. Electroencephalogr Clin Neurophysiol 52: 494–496. - PubMed
- Calford MB, Tweedale R (1990) Interhemispheric transfer of plasticity in the cerebral cortex. Science 249: 805–807. - PubMed
- Conti F, Fabri M, Manzoni T (1986) Bilateral receptive fields and callosal connectivity of the body midline representation in the first somotosensory area of primates. Somatosens Res 3: 273–289. - PubMed
- Disbrow E, Roberts T, Poeppel D, Krubitzer L (2001) Evidence for inter-hemispheric processing of inputs from the hands in human S2 and PV. J Neurophysiol 85: 2236–2244. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources