Neuronal or glial expression of human apolipoprotein e4 affects parenchymal and vascular amyloid pathology differentially in different brain regions of double- and triple-transgenic mice - PubMed (original) (raw)
Comparative Study
Neuronal or glial expression of human apolipoprotein e4 affects parenchymal and vascular amyloid pathology differentially in different brain regions of double- and triple-transgenic mice
Tom Van Dooren et al. Am J Pathol. 2006 Jan.
Abstract
Apolipoprotein E4 (ApoE4) is associated with Alzheimer's disease by unknown mechanisms. We generated six transgenic mice strains expressing human ApoE4 in combination with mutant amyloid precursor protein (APP) and mutant presenilin-1 (PS1) in single-, double-, or triple-transgenic combinations. Diffuse, but not dense, amyloid plaque-load in subiculum and cortex was increased by neuronal but not glial ApoE4 in old (15 months) double-transgenic mice, whereas both diffuse and dense plaques formed in thalamus in both genotypes. Neuronal and glial ApoE4 promoted cerebral amyloid angiopathy as extensively as mutant PS1 but with pronounced regional differences: cortical angiopathy was induced by neuronal ApoE4 while thalamic angiopathy was again independent of ApoE4 source. Angiopathy correlated more strongly with soluble Abeta40 and Abeta42 levels in cortex than in thalamus throughout the six genotypes. Neither neuronal nor glial ApoE4 affected APP proteolytic processing, as opposed to mutant PS1. Neuronal ApoE4 increased soluble amyloid levels more than glial ApoE4, but the Abeta42/40 ratios were similar, although significantly higher than in single APP transgenic mice. We conclude that although the cellular origin of ApoE4 differentially affects regional amyloid pathology, ApoE4 acts on the disposition of amyloid peptides downstream from their excision from APP but without induction of tauopathy.
Figures
Figure 1
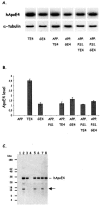
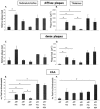
Expression levels of human ApoE4 and neuron-specific fragmentation. A: Western blotting of total brain extracts of mice with the six genotypes as indicated for human ApoE and for murine α-tubulin, as loading control. B: Concentrations of human ApoE4 in brain of the six strains of transgenic mice (mean ± SEM, n = 4) generated and analyzed in this study and of the two parental strains, ie, thy1-ApoE4 and GFAP-ApoE4. Human ApoE4 is evidently absent in the parental APP mice and in the APP.PS1 mice. Note that for all of the other genotypes the mice are hemizygous for ApoE4, except for the parental thy1-ApoE4 mice that are homozygous for ApoE4. C: Western blotting showing human ApoE4 and the 16-kd proteolytic fragment (arrow) in the brain of the following transgenic mice: 1, APP; 2, thy1-ApoE4; 3, GFAP-ApoE4; 4, APP.PS1; 5, APP.TE4; 6, APP.GE4; 7, APP.PS1.TE4; 8, APP.PS1.GE4. Note that the 16-kd fragment is only observed in brains of mice that express human ApoE4 in neurons, and that these also appear to contain a fragment of ∼26 kd (see Results for details).
Figure 2
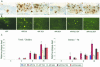
Amyloid plaque-load in subiculum. A and B: Staining with mAb 3D6 (A) and with thioflavinS (B) of the subiculum of the six transgenic mouse strains (age, 15 months). C: Quantification of relative total and dense plaque-load expressed as surface area covered by plaques (percent; mean ± SEM, n = 3 or 4 per genotype and per time point) in the six genotypes at ages 4, 6, 8, and 15 months as indicated on the ordinate. Asterisks denote statistical differences at P < 0.05. Scale bars, 100 μm.
Figure 3
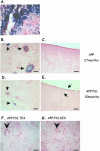
Amyloid pathology in thalamus. A and B: Staining with mAb 3D6 (A) and with thioflavinS (B) of the subiculum of the six transgenic mouse strains (age, 15 months). C: Quantification of absolute levels (μm2) of plaque-load (mean ± SEM) in 15-month-old mice. *P < 0.05. D: Frequency of presence of diffuse plaques (mAb 3D6) in thalamus: 0 = not present; 1 = present in all three sections analyzed per mouse. E: Immunostaining with polyclonal antibody PanAβ and with mAb 6E10 detects intraneuronal amyloid in hippocampus and cortex but not in thalamus of APP.PS1.TE4 triple-transgenic mouse. Arrows indicate location of amyloid plaques. Scale bars: 200 μm (A, B); 400 μm (E, hippocampus); 50 μm (E, cortex and thalamus).
Figure 4
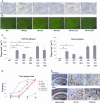
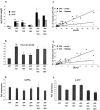
Amyloid angiopathy (CAA) in cortex and thalamus. A–D: Representative images of vascular amyloid in cortex (A, C) and thalamus (B, D) of an APP.PS1.GE4 triple-transgenic mouse stained with thioflavinS and with X34. E: Quantification of CAA in cortex and thalamus, expressed as number of positive blood vessels per three sections (mean ± SEM) of the six genotypes in function of age (4, 6, 8, and 15 months). Asterisks denote statistical significance at P < 0.05. Scale bars: 50 μm (A, B); 100 μm (C, D).
Figure 5
Amyloid pathology in transgenic mice of the six genotypes at age 15 months. For clarity and ease of comparison, this figure compiles all data of the amyloid pathology, ie, diffuse and dense plaques and angiopathy as indicated, in the oldest transgenic mice with the six genotypes (age, 15 months). The left panels represent subiculum and cortex, and right panels contain data for the thalamus as indicated. Asterisks denote statistical significance at P < 0.05 between groups connected by the horizontal bars (mean ± SEM).
Figure 6
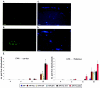
Histochemical staining for non-heme iron as index of microhemorrhages. A: Representative image of mouse spleen stained for non-heme iron (blue) as positive control. B and C: Microbleedings (arrows) in thalamus (B) but not in cortex (C) of very old APP transgenic mouse (27 months) included as positive control. D and E: Microbleedings in thalamus (D, arrows) and cortical meninges (E, arrows) of a very old APP.PS1 mouse (30 months) included as positive control. F and G: Staining for non-heme iron of thalamus of an APP.PS1.TE4 and APP.PS1.GE4 mouse. Note that no microhemorrhages were observed in thalamus or cortical meninges of any of the old transgenic mice analyzed in the current study (see text for details and discussion). Arrowhead, blood vessel. Scale bars: 50 μm (thalamus); 100 μm (cortex).
Figure 7
Biochemical analysis of amyloid peptides and processing of APP in brain. A and B: Levels of soluble Aβ40 and Aβ42 (A) and the Aβ42/40 ratio (B), in brain of old transgenic mice (15 months) measured by specific sandwich ELISAs (mean ± SEM, n = 3 or 4). Asterisks denote statistical significance at *P < 0.05, **P < 0.01, ***P < 0.001. C and D: Correlation between Aβ40 (C) and Aβ42 (D) levels with cortical and thalamic CAA over all genotypes analyzed in this study. Spearman rho: (Aβ40 versus CAA-cortex: r = 0.766), (Aβ40 versus CAA-thalamus: r = 0.606), (Aβ42 versus CAA-cortex: r = 0.821), and (Aβ42 versus CAA-thalamus: r = 0.657); all P < 0.01. E and F: Levels of total mature human APP (E) and of β-CTF (C99) (F) in brain of all transgenic mice at age 15 months.
Similar articles
- Prominent cerebral amyloid angiopathy in transgenic mice overexpressing the london mutant of human APP in neurons.
Van Dorpe J, Smeijers L, Dewachter I, Nuyens D, Spittaels K, Van Den Haute C, Mercken M, Moechars D, Laenen I, Kuiperi C, Bruynseels K, Tesseur I, Loos R, Vanderstichele H, Checler F, Sciot R, Van Leuven F. Van Dorpe J, et al. Am J Pathol. 2000 Oct;157(4):1283-98. doi: 10.1016/S0002-9440(10)64644-5. Am J Pathol. 2000. PMID: 11021833 Free PMC article. - Lack of tau proteins rescues neuronal cell death and decreases amyloidogenic processing of APP in APP/PS1 mice.
Leroy K, Ando K, Laporte V, Dedecker R, Suain V, Authelet M, Héraud C, Pierrot N, Yilmaz Z, Octave JN, Brion JP. Leroy K, et al. Am J Pathol. 2012 Dec;181(6):1928-40. doi: 10.1016/j.ajpath.2012.08.012. Epub 2012 Sep 28. Am J Pathol. 2012. PMID: 23026200 - Human apolipoprotein E2 promotes parenchymal amyloid deposition and neuronal loss in vasculotropic mutant amyloid-β protein Tg-SwDI mice.
Xu F, Vitek MP, Colton CA, Previti ML, Davis J, Van Nostrand WE. Xu F, et al. J Alzheimers Dis. 2012;31(2):359-69. doi: 10.3233/JAD-2012-120421. J Alzheimers Dis. 2012. PMID: 22635103 - Modeling Alzheimer's disease in transgenic mice: effect of age and of presenilin1 on amyloid biochemistry and pathology in APP/London mice.
Dewachter I, van Dorpe J, Spittaels K, Tesseur I, Van Den Haute C, Moechars D, Van Leuven F. Dewachter I, et al. Exp Gerontol. 2000 Sep;35(6-7):831-41. doi: 10.1016/s0531-5565(00)00149-2. Exp Gerontol. 2000. PMID: 11053674 Review. - Cerebral amyloid angiopathy and dementia.
Tian J, Shi J, Mann DM. Tian J, et al. Panminerva Med. 2004 Dec;46(4):253-64. Panminerva Med. 2004. PMID: 15876981 Review.
Cited by
- Tg-SwDI transgenic mice exhibit novel alterations in AbetaPP processing, Abeta degradation, and resilient amyloid angiopathy.
Van Vickle GD, Esh CL, Daugs ID, Kokjohn TA, Kalback WM, Patton RL, Luehrs DC, Walker DG, Lue LF, Beach TG, Davis J, Van Nostrand WE, Castaño EM, Roher AE. Van Vickle GD, et al. Am J Pathol. 2008 Aug;173(2):483-93. doi: 10.2353/ajpath.2008.071191. Epub 2008 Jul 3. Am J Pathol. 2008. PMID: 18599612 Free PMC article. - Genetic deletion of TNFRII gene enhances the Alzheimer-like pathology in an APP transgenic mouse model via reduction of phosphorylated IκBα.
Jiang H, He P, Xie J, Staufenbiel M, Li R, Shen Y. Jiang H, et al. Hum Mol Genet. 2014 Sep 15;23(18):4906-18. doi: 10.1093/hmg/ddu206. Epub 2014 May 13. Hum Mol Genet. 2014. PMID: 24824215 Free PMC article. - Cell-specific production, secretion, and function of apolipoprotein E.
Kockx M, Traini M, Kritharides L. Kockx M, et al. J Mol Med (Berl). 2018 May;96(5):361-371. doi: 10.1007/s00109-018-1632-y. Epub 2018 Mar 7. J Mol Med (Berl). 2018. PMID: 29516132 Review. - Human apolipoprotein E isoforms are differentially sialylated and the sialic acid moiety in ApoE2 attenuates ApoE2-Aβ interaction and Aβ fibrillation.
Moon HJ, Haroutunian V, Zhao L. Moon HJ, et al. Neurobiol Dis. 2022 Mar;164:105631. doi: 10.1016/j.nbd.2022.105631. Epub 2022 Jan 15. Neurobiol Dis. 2022. PMID: 35041991 Free PMC article. - Long-term treatment of thalidomide ameliorates amyloid-like pathology through inhibition of β-secretase in a mouse model of Alzheimer's disease.
He P, Cheng X, Staufenbiel M, Li R, Shen Y. He P, et al. PLoS One. 2013;8(2):e55091. doi: 10.1371/journal.pone.0055091. Epub 2013 Feb 6. PLoS One. 2013. PMID: 23405115 Free PMC article.
References
- Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. - PubMed
- Dewachter I, Van Leuven F. Secretases as targets for the treatment of Alzheimer’s disease: the prospects. Lancet Neurol. 2002;1:409–416. - PubMed
- Selkoe DJ. Defining molecular targets to prevent Alzheimer disease. Arch Neurol. 2005;2:192–195. - PubMed
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. - PubMed
- Bales KR, Dodart JC, DeMattos RB, Holtzman DM, Paul SM. Apolipoprotein E, amyloid, and Alzheimer disease. Mol Interv. 2002;6:363–375. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases