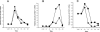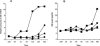Adrenomedullin is a cross-talk molecule that regulates tumor and mast cell function during human carcinogenesis - PubMed (original) (raw)
. 2006 Jan;168(1):280-91.
doi: 10.2353/ajpath.2006.050291.
Alfredo Martínez, Mercedes Garayoa, Rubén Pío, Gurmeet Kaur, Michael R Woolhiser, Dean D Metcalfe, William A Hook, Reuben P Siraganian, Theresa A Guise, John M Chirgwin, Frank Cuttitta
Affiliations
- PMID: 16400030
- PMCID: PMC1592665
- DOI: 10.2353/ajpath.2006.050291
Adrenomedullin is a cross-talk molecule that regulates tumor and mast cell function during human carcinogenesis
Enrique Zudaire et al. Am J Pathol. 2006 Jan.
Abstract
We have previously demonstrated that adrenomedullin (AM) plays a critical role as an autocrine/paracrine tumor cell survival factor. We now present evidence that AM is an important regulator of mast cell (MC) function and that this modulation is potentially involved in tumor promotion. AM induced histamine or beta-hexosaminidase release from rat and human MCs through a receptor-independent pathway. AM was chemotactic for human MCs and stimulated mRNA expression of vascular endothelial growth factor, monocyte chemoattractant protein-1, and basic fibroblast growth factor in this cell type. Differentiated but not undifferentiated human MCs responded to hypoxic insult with elevated AM mRNA/protein expression. Using confocal microscopy, we identified AM-producing MCs in tumor infiltrates of human breast and lung cancer patients. In mixed culture assays the AM-producing human MC line HMC-1 augmented both anchorage-dependent and -independent growth of human lung cancer A549 cells, an effect that was suppressed by MC-targeted siRNA AM knockdown. Finally, HMC-1 cells induced in vivo angiogenesis as assessed by directed in vivo angiogenesis assay analysis; neutralizing anti-AM monoclonal antibody blocked this ability. Our collective data suggest a new role for AM as a cross-talk molecule that integrates tumor and MC communication, underlying a unique promotion mechanism of human cancers.
Figures
Figure 1
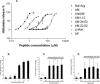
Peptide-induced histamine (HA) or β-hex release from MCs. A: The resulting dose-response curves measure the ability of different peptides to induce histamine release from isolated rat peritoneal MCs. The neutralizing anti-AM MoAb-G6 (10 μg/ml; filled inverted triangles) had no effect on AM-mediated histamine release (filled triangles). Peptide fragments AM1-12 (diamonds), AM34-52 (circles), and AM22-52 (open squares) had virtually no effect in eliciting MC degranulation. Increases in net charge tracks with the ability of a given peptide to induce histamine release as demonstrated by comparing Nal-Arg (filled squares) to LHRH (open triangles) results. Each point represents the mean and SD of five independent repeats. B: Dose-response curve of AM-induced β-hex release from HCMCs. C: Effect of varying concentration of anti-AM MoAb-G6 on AM (100 μmol/L)-mediated HCMC degranulation. D: Complement factor H effect on AM (100 μmol/L)-induced β-hex release in HCMCs. Each bar represents the mean and SD of four independent repeats. ns, Not statistically significant; ***P < 0.001.
Figure 2
AM functions as a chemotactic factor of human MC. A: Dose-dependent modulation of hMC migration by AM. Each bar represents the mean and SD of three independent repeats. *P < 0.05 and **P < 0.01. B: HMC-1 control migration through porous filter. C: HMC-1 migration through porous filter in the presence of 1 nmol/L AM. D: Cross-section of Matrigel plug containing AM from DIVAA analysis, which identifies MC infiltrates (red stain) at the site of newly formed blood vessels. Scale bar, 50 μm; 25 μm (inset).
Figure 3
AM stimulates the expression of MC proangiogenic factors. A: AM (100 nmol/L) exposure causes a time-dependent increase in VEGF mRNA expression in differentiated (+PMA, diamonds) and undifferentiated (−PMA, squares) HMC-1 cells. B: AM-induced MCP-1 expression in HMC-1 cells. C: AM-mediated bFGF expression in HMC-1 cells. The y axis represents relative mRNA induction for a given angiogenic factor when comparing plus/minus AM treatment.
Figure 4
Hypoxia up-regulation of AM mRNA/peptide expression in human MCs. A: Reduced oxygen tension (1.0% O2) caused a dramatic increase in AM mRNA expression in differentiated HMC-1 cells (squares) throughout a 48-hour interim while having no effect on undifferentiated HMC-1 cells (circles) throughout the same time period. AM mRNA normoxic response data for differentiated (diamonds) and undifferentiated (triangles) HMC-1 cells is also presented. B: Time-dependent release of immunoreactive AM detected in the conditioned media of HMC-1 cells incubated under normoxic or hypoxic conditions (see symbols above for treatment identification).
Figure 5
Immunohistochemical detection of AM-containing MCs in human cancer tissue. A–D: Archival material of human breast cancer; E–H: pathological specimens of human lung adenocarcinoma. Red fluorescence is indicative of AM immunostaining (A and E), green deposition represents localization of tryptase-containing MCs (B and F), blue coloration is 4,6-diamidino-2-phenylindole staining of cellular nuclei (C and G), and yellowish staining indicates co-localization of AM and tryptase in MCs (arrows in D and H). tc, Tumor cell; br, bronchiolus. Scale bar, 15 μm.
Figure 6
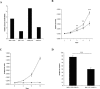
MC-derived AM enhances A549 anchorage-dependent and -independent growth. A: AM mRNA evaluation by quantitative real-time PCR of HMC-1 and A549 cells expressing a siRNA for AM or scrambled sequence. A significant down-regulation of at least 65% was observed in both cells lines. B: MTT growth assay showing the effect of conditioned media from HMC-1-SCR and HMC-1-511 on A549-511 growth. A549-511 was able to grow significantly faster in the presence of conditioned media from HMC-1-SCR (diamonds) than HMC-1-511 (squares). C: MTT growth assay showing complete absence of growth in HMC-1 after treatment with PMA. D: Comparison of the anchorage-independent growth of A549-511 in co-culture with PMA-treated HMC-1-SCR or HMC-1-511 in clonogenic assays. A549-511 cloned better in the presence of HMC-1-SCR than when co-cultured with HMC-1-511. **P < 0.01 and ***P < 0.001.
Figure 7
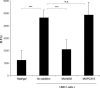
MC-derived AM functions as an in vivo angiogenic factor. HMC-1 (104 cells/angioreactor) induces a statistically significant increase (P < 0.001) in new blood vessel formation as compared to the empty angioreactor when evaluated by DIVAA analysis. The addition of 0.8 μg/ml of MoAb-G6 markedly suppressed (P < 0.001) HMC-1-induced angiogenesis demonstrating AM dependence of vessel formation. An indifferent isotopic control antibody (MOPC315) was ineffective in suppressing the response. Relative fluorescence unit (RFU) values are directly proportional to the amount of new blood vessels formed for a given experimental sample. Each bar represents the mean and SD of six independent repeats. *** P < 0.001.
Figure 8
Proposed model of the AM/tumor cell/MC relationship in human carcinogenesis. Tumor-derived AM is released into the microenvironment setting up a concentration gradient of peptide that attracts distal MCs to infiltrate the tumor site. As MCs migrate up the peptide gradient, higher AM concentrations are reached, slowing MC migration and stimulating MC-derived angiogenic factor expression and ultimately MC release at the tumor site. Given that the microenvironment around tumor cells tends to be hypoxic, this reduced oxygen state would also augment MC-derived AM expression/release. MC-derived AM could mediate a paracrine tumor survival effect (direct mitogen, angiogenic factor, and anti-apoptosis), initiate an autocrine loop-based relationship, or function as a paracrine recruitment factor drawing additional MCs to the area, thus perpetuating the inflammatory process and enhancing tumor promotion.
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924 - Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.
Love AMA, Edwards C, Cai RY, Gibbs V. Love AMA, et al. Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article. - A Blog-Based Study of Autistic Adults' Experiences of Aloneness and Connection and the Interplay with Well-Being: Corpus-Based and Thematic Analyses.
Petty S, Allen S, Pickup H, Woodier B. Petty S, et al. Autism Adulthood. 2023 Dec 1;5(4):437-449. doi: 10.1089/aut.2022.0073. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116056 Free PMC article. - Antioxidants for female subfertility.
Showell MG, Mackenzie-Proctor R, Jordan V, Hart RJ. Showell MG, et al. Cochrane Database Syst Rev. 2017 Jul 28;7(7):CD007807. doi: 10.1002/14651858.CD007807.pub3. Cochrane Database Syst Rev. 2017. PMID: 28752910 Free PMC article. Updated. Review.
Cited by
- Transcriptional regulation of adrenomedullin by oncostatin M in human astroglioma cells: implications for tumor invasion and migration.
Lim SY, Ahn SH, Park H, Lee J, Choi K, Choi C, Choi JH, Park EM, Choi YH. Lim SY, et al. Sci Rep. 2014 Sep 23;4:6444. doi: 10.1038/srep06444. Sci Rep. 2014. PMID: 25246098 Free PMC article. - Mast Cells in Lung Homeostasis: Beyond Type I Hypersensitivity.
Campillo-Navarro M, Chávez-Blanco AD, Wong-Baeza I, Serafín-López J, Flores-Mejía R, Estrada-Parra S, Estrada-García I, Chacón-Salinas R. Campillo-Navarro M, et al. Curr Respir Med Rev. 2014 Jun;10(2):115-123. doi: 10.2174/1573398X10666141024220151. Curr Respir Med Rev. 2014. PMID: 25484639 Free PMC article. - Integrated analysis of single-cell and bulk RNA-sequencing identifies a metastasis-related gene signature for predicting prognosis in lung adenocarcinoma.
Cao X, Xi J, Wang C, Yu W, Wang Y, Zhu J, Xu K, Pan D, Chen C, Han Z. Cao X, et al. Clin Transl Oncol. 2024 Nov 8. doi: 10.1007/s12094-024-03752-6. Online ahead of print. Clin Transl Oncol. 2024. PMID: 39516442 - Mast cells and angiogenesis in pancreatic ductal adenocarcinoma.
Longo V, Tamma R, Brunetti O, Pisconti S, Argentiero A, Silvestris N, Ribatti D. Longo V, et al. Clin Exp Med. 2018 Aug;18(3):319-323. doi: 10.1007/s10238-018-0493-6. Epub 2018 Feb 28. Clin Exp Med. 2018. PMID: 29492715 Review. - Peptidergic Systems and Cancer: Focus on Tachykinin and Calcitonin/Calcitonin Gene-Related Peptide Families.
Sánchez ML, Rodríguez FD, Coveñas R. Sánchez ML, et al. Cancers (Basel). 2023 Mar 9;15(6):1694. doi: 10.3390/cancers15061694. Cancers (Basel). 2023. PMID: 36980580 Free PMC article. Review.
References
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
- Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends Genet. 1993;9:138–141. - PubMed
- Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Huntingt) 2002;16:217–230. - PubMed
- Ribatti D, Vacca A, Nico B, Crivellato E, Roncali L, Dammacco F. The role of mast cells in tumour angiogenesis. Br J Haematol. 2001;115:514–521. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials