The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation - PubMed (original) (raw)
The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation
Jeffrey C Nolz et al. Curr Biol. 2006.
Abstract
Background: The engagement of the T cell receptor results in actin cytoskeletal reorganization at the immune synapse (IS) and the triggering of biochemical signaling cascades leading to gene regulation and, ultimately, cellular activation. Recent studies have identified the WAVE family of proteins as critical mediators of Rac1-induced actin reorganization in other cell types. However, whether these proteins participate in actin reorganization at the IS or signaling pathways in T cells has not been investigated.
Results: By using a combination of biochemical, genetic, and cell biology approaches, we provide evidence that WAVE2 is recruited to the IS, is biochemically modified, and is required for actin reorganization and beta-integrin-mediated adhesion after TCR crosslinking. Moreover, we show that WAVE2 regulates calcium entry at a point distal to PLCgamma1 activation and IP(3)-mediated store release.
Conclusions: These data reveal a role for WAVE2 in regulating multiple pathways leading to T cell activation. In particular, this work shows that WAVE2 is a key component of the actin regulatory machinery in T cells and that it also participates in linking intracellular calcium store depletion to calcium release-activated calcium (CRAC) channel activation.
Figures
Figure 1
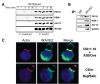
The WAVE2 Complex Localizes to the IS (A) Jurkat T cells were stimulated for indicated time points (above lanes). After stimulation, cells were lysed, clarified, and subsequently immunoprecipitated with control IgG antibody or antibody against WAVE2. IPs were separated by SDS-PAGE, transferred, and subsequently blotted for PIR121, HEM-1, Abi-1/2, and WAVE2. (B) Primary CD4+ T cells were isolated, lysed, immunoprecipitated, and subsequently blotted as in (A). (C) Ovalbumin peptide-pulsed A20 B cells (blue) were incubated with secondary DO11.10 transgenic T cells (top). Conjugated cells were bound to poly-L-lysine coated coverslips, fixed, and stained with antibody against WAVE2 (green) and rhodamine-phalloidin (red). Also shown are primary CD4+ T cells conjugated to superantigen cocktail (SAC) pulsed Raji B cells (bottom).
Figure 2
Depletion of WAVE2 Leads to Subsequent Degradation of Other Members of the Complex (A) Three subsequent IPs for WAVE2 were performed on 0.5 mg of Jurkat T cell lysate (WAVE2#1-3, lanes 1–3). After preclearing the lysate of any residual WAVE2 antibody (bead clear, lane 4), an IP for HEM-1 and PIR121 was performed (PIR121/HEM-1, lane 5). HEM-1 and PIR121 was also directly IP’ed from 0.5 mg of Jurkat T cell lysate (PIR121/HEM-1, lane 6). Bound proteins from each IP were separated by SDS-PAGE, transferred, and subsequently blotted for WAVE2, HEM-1, and PIR121. (B) Jurkat T cells were transfected with a control vector (vector) or one of two shWAVE2 suppression vectors (shWAVE2-A/-B). Cells were lysed at the 24, 48, and 72 hr time points. Equal amounts of protein were separated by SDS-PAGE, transferred, and blotted for WAVE2 and ZAP-70. (C) Cells were transfected as in (B) with the addition of two suppression vectors against HEM-1 (shHEM-1-A/-B). After 72 hr, cells were harvested as in (B) and blotted for PIR121, HEM-1, Abi-2, WAVE2, and ZAP-70.
Figure 3
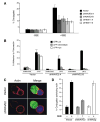
Suppression of WAVE2 Decreases Conjugate Formation and Actin Accumulation at the IS (A) Cells were transfected with either vector control or suppression vectors targeting either WAVE2 or HEM-1. After 72 hr, transfected cells were incubated with NALM6 B cells stained red with ethidium bromide and pulsed with SEE (+SEE) or left unpulsed (−SEE). Using GFP expression as a marker for transfected cells, conjugate formation was determined using two-color flow cytometry. Shown is a representative example of three independent experiments. (B) Jurkat T cells were transfected as in (A) and were analyzed for their ability to bind to fibronectin following no stimulation (unstimulated) or stimulation of the TCR (OKT3) for 10 min at 37ºC.After Following washing, adherent cells were collected and the adhesion of GFP-low, intermediate, and high cells in each sample was determined by flow cytometry. (C) Cells were transfected as in (A) and after 72 hr were incubated with SEE-pulsed NALM6 B cells (blue). Conjugates were bound to poly-L-lysine coated coverslips, fixed, and stained with rhodamine-phalloidin (red) to visualize the accumulation of F-actin. (D) Conjugates formed in (C) and Figure S4 were quantified on their ability to form F-actin at the IS. Shown is the average of three independent experiments.
Figure 4
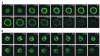
WAVE2-Suppressed T Cells Fail to Spread Properly in Response to TCR Ligation Jurkat T cells stably expressing GFP-actin were transfected with (A) control shRNA-cherry vector or (B) cherry-shWAVE2-B. After 48 hr, the cells were placed onto OKT3 mAb-coated coverslips and activation events were recorded by taking images every 5 s, as explained in the Experimental Procedures. Shown are representative time-lapsed images occurring after initial contact with the OKT3-coated coverslips. More than 100 cherry-expressing cells were analyzed for each transfection. Images were taken at the same magnification. Movies are available online with the Supplemental Data.
Figure 5
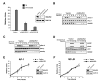
WAVE2 Regulates T Cell Activation Independently of PLCγ1, Erk, and Jnk Activation (A) Jurkat T cells were transfected with IL-2p luciferase reporter along with either a control vector or one of two shWAVE2 suppression vectors (shWAVE2-A/-B). After 72 hr, cells were left unstimulated (Nil) or stimulated with OKT3 and anti-CD28 for 5 hr. Luciferase activity was measured and normalized to TK-Renilla readings. (B) Jurkat T cells were transfected with vector control or shWAVE2 suppression vector. After 72 hr, cells were stimulated with OKT3 and goat anti-mouse antibody for the indicated time points. Whole-cell extracts were prepared and proteins were separated by SDS-PAGE, transferred, and analyzed for phosphorylation of PLCγ1 (Tyr 783) by immunoblot. (C and D) Same as in (B) but blotted for phosphorylation of Erk1 and Erk2 and Jnk1 and Jnk2. (E and F) Cells were transfected as in (A), but with a reporter construct for (E) AP-1 or (F) NFκB. Cells were stimulated for 2, 3, 4, or 5 hr, and luciferase activity was measured and normalized to TK-Renilla readings. Data shown are representative examples of three independent experiments for each.
Figure 6
WAVE2 Regulates CRAC Channel Activation Leading to NFAT-Mediated Gene Transcription (A) Jurkat T cells were transfected with vector control or shWAVE2 suppression vector. After 72 hr, calcium mobilization in response to OKT3/ goat anti-mouse was measured using Indo-1 staining and flow cytometry. (B) Jurkat T cells were transfected as in (A). After 72 hr, calcium measurements were performed using single cell fluorescence ratio (Fura-2 imaging) of GFP positive control and WAVE2 suppressed Jurkat cells. In calcium-free bath solution, increases in Fura-2 ratio reflect Ca2+ release from intracellular stores. Extracellular Ca2+ entry via activated CRAC channels was subsequently assessed by reintroduction of extracellular calcium (2 mM Ca2+). Shown are representative examples of three independent experiments for each condition and each trace represents the average response of at least 100 cells in the recording chamber. (C and D) Cells were prepared and analyzed as in (B) and stimulated as indicated above the graphs. (E and F) Jurkat T cells were transfected with vector control or shWAVE2 suppression vector and either a (E) NFAT/AP-1 or (F) NFAT luciferase reporter. 72 hr post transfection, cells were stimulated with OKT3 and anti-CD28 for 2, 3, 4, and 5 hr. Luciferase activity was measured at each time point and normalized to TK-Renilla readings. Shown are representative examples of three independent experiments for each.
Similar articles
- Role for the Abi/wave protein complex in T cell receptor-mediated proliferation and cytoskeletal remodeling.
Zipfel PA, Bunnell SC, Witherow DS, Gu JJ, Chislock EM, Ring C, Pendergast AM. Zipfel PA, et al. Curr Biol. 2006 Jan 10;16(1):35-46. doi: 10.1016/j.cub.2005.12.024. Curr Biol. 2006. PMID: 16401422 - WAVE2 regulates high-affinity integrin binding by recruiting vinculin and talin to the immunological synapse.
Nolz JC, Medeiros RB, Mitchell JS, Zhu P, Freedman BD, Shimizu Y, Billadeau DD. Nolz JC, et al. Mol Cell Biol. 2007 Sep;27(17):5986-6000. doi: 10.1128/MCB.00136-07. Epub 2007 Jun 25. Mol Cell Biol. 2007. PMID: 17591693 Free PMC article. - The c-Abl tyrosine kinase regulates actin remodeling at the immune synapse.
Huang Y, Comiskey EO, Dupree RS, Li S, Koleske AJ, Burkhardt JK. Huang Y, et al. Blood. 2008 Jul 1;112(1):111-9. doi: 10.1182/blood-2007-10-118232. Epub 2008 Feb 27. Blood. 2008. PMID: 18305217 Free PMC article. - Coordinate control of cytoskeletal remodeling and calcium mobilization during T-cell activation.
Babich A, Burkhardt JK. Babich A, et al. Immunol Rev. 2013 Nov;256(1):80-94. doi: 10.1111/imr.12123. Immunol Rev. 2013. PMID: 24117814 Free PMC article. Review. - T-cell-receptor-dependent actin regulatory mechanisms.
Huang Y, Burkhardt JK. Huang Y, et al. J Cell Sci. 2007 Mar 1;120(Pt 5):723-30. doi: 10.1242/jcs.000786. J Cell Sci. 2007. PMID: 17314246 Review.
Cited by
- T cell antigen receptor activation and actin cytoskeleton remodeling.
Kumari S, Curado S, Mayya V, Dustin ML. Kumari S, et al. Biochim Biophys Acta. 2014 Feb;1838(2):546-56. doi: 10.1016/j.bbamem.2013.05.004. Epub 2013 May 14. Biochim Biophys Acta. 2014. PMID: 23680625 Free PMC article. Review. - Pathogen-specific inflammatory milieux tune the antigen sensitivity of CD8(+) T cells by enhancing T cell receptor signaling.
Richer MJ, Nolz JC, Harty JT. Richer MJ, et al. Immunity. 2013 Jan 24;38(1):140-52. doi: 10.1016/j.immuni.2012.09.017. Epub 2012 Dec 20. Immunity. 2013. PMID: 23260194 Free PMC article. - Actin engine in immunological synapse.
Piragyte I, Jun CD. Piragyte I, et al. Immune Netw. 2012 Jun;12(3):71-83. doi: 10.4110/in.2012.12.3.71. Epub 2012 Jun 30. Immune Netw. 2012. PMID: 22916042 Free PMC article. - Asef2 functions as a Cdc42 exchange factor and is stimulated by the release of an autoinhibitory module from a concealed C-terminal activation element.
Hamann MJ, Lubking CM, Luchini DN, Billadeau DD. Hamann MJ, et al. Mol Cell Biol. 2007 Feb;27(4):1380-93. doi: 10.1128/MCB.01608-06. Epub 2006 Dec 4. Mol Cell Biol. 2007. PMID: 17145773 Free PMC article. - Modular design of immunological synapses and kinapses.
Dustin ML. Dustin ML. Cold Spring Harb Perspect Biol. 2009 Jul;1(1):a002873. doi: 10.1101/cshperspect.a002873. Cold Spring Harb Perspect Biol. 2009. PMID: 20066081 Free PMC article. Review.
References
- Holsinger LJ, Graef IA, Swat W, Chi T, Bautista DM, Davidson L, Lewis RS, Alt FW, Crabtree GR. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr Biol. 1998;8:563–572. - PubMed
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. - PubMed
- Cantrell DA. GTPases and T cell activation. Immunol Rev. 2003;192:122–130. - PubMed
- Thrasher AJ. WASp in immune-system organization and function. Nat Rev Immunol. 2002;2:635–646. - PubMed
- Cannon JL, Burkhardt JK. Differential roles for Wiskott-Aldrich syndrome protein in immune synapse formation and IL-2 production. J Immunol. 2004;173:1658–1662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-AI31126R01-AI384/AI/NIAID NIH HHS/United States
- R01-AI065474/AI/NIAID NIH HHS/United States
- R01-AI39678/AI/NIAID NIH HHS/United States
- R01 AI065474/AI/NIAID NIH HHS/United States
- R01-AI44835/AI/NIAID NIH HHS/United States
- R01-AI060921/AI/NIAID NIH HHS/United States
- R01 AI031126/AI/NIAID NIH HHS/United States
- R01 AI060921/AI/NIAID NIH HHS/United States
- R01 AI044835/AI/NIAID NIH HHS/United States
- R01 AI038474/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials