Monoamine oxidase: isoforms and inhibitors in Parkinson's disease and depressive illness - PubMed (original) (raw)
Review
Monoamine oxidase: isoforms and inhibitors in Parkinson's disease and depressive illness
Moussa B H Youdim et al. Br J Pharmacol. 2006 Jan.
Abstract
A few years after the foundation of the British Pharmacological Society, monoamine oxidase (MAO) was recognized as an enzyme of crucial interest to pharmacologists because it catalyzed the major inactivation pathway for the catecholamine neurotransmitters, noradrenaline, adrenaline and dopamine (and, later, 5-hydroxytryptamine, as well). Within the next decade, the therapeutic value of inhibitors of MAO in the treatment of depressive illness was established. Although this first clinical use exposed serious side effects, pharmacological interest in, and investigation of, MAO continued, resulting in the characterization of two isoforms, MAO-A and -B, and isoform-selective inhibitors. Selective inhibitors of MAO-B have found a therapeutic role in the treatment of Parkinson's disease and further developments have provided reversible inhibitors of MAO-A, which offer antidepressant activity without the serious side effects of the earlier inhibitors. Clinical observation and subsequent pharmacological analysis have also generated the concept of neuroprotection, reflecting the possibility of slowing, halting and maybe reversing, neurodegeneration in Parkinson's or Alzheimer's diseases. Increased levels of oxidative stress in the brain may be critical for the initiation and progress of neurodegeneration and selective inhibition of brain MAO could contribute importantly to lowering such stress. There are complex interactions between free iron levels in brain and MAO, which may have practical outcomes for depressive disorders. These aspects of MAO and its inhibition and some indication of how this important area of pharmacology and therapeutics might develop in the future are summarized in this review.
Figures
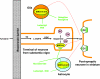
Figure 1
The ‘cheese reaction' – potentiation of cardiovascular effects of tyramine (or other indirectly acting sympathomimetic amines) by irreversible inhibitors of MAO. Normally, dietary tyramine suffers extensive ‘first pass' inactivation by the MAO isoforms in gut wall and then in the liver. The tyramine that survives to enter the systemic circulation is further attenuated by the MAO in vascular endothelial cells and lung (Bakhle, 1990). At the adrenergic neurone, uptake of tyramine initiates the release of noradrenaline, which accounts for the sympathomimetic effects of tyramine. Irreversible inhibition of MAO-A, the predominant isoform in the periphery, allows greatly increased amounts of tyramine to enter the systemic circulation and, from there, adrenergic neurons, consequently increasing noradrenaline release and effect. By contrast, reversible inhibitors of MAO-A (RIMAs) are displaced from the enzyme by tyramine which is then metabolized normally by the enzyme. Thus circulating tyramine never attains the high levels resulting from irreversible inhibition of MAO.
Figure 2
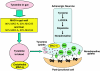
The crystal structure of human MAO-B. There are three functionally distinct domains, as shown. In red, the substrate domain contains two ‘cavities' shown in cyan. The outer space is the entrance cavity leading to the inner space, substrate binding cavity, closer to the flavin cofactor. The flavin-binding domain is shown in blue with the FAD molecule in yellow. In green, the C-terminal helical region which attaches the protein to the mitochondrial membrane. Rasagiline covalently links to the flavin via its propargylamine group (yellow arrow) and the indan ring then extends into the substrate-binding cavity, blocking access for substrate.
Figure 3
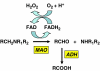
Reaction pathway of monoamine metabolism by oxidative deamination by mitochondrial MAO. The primary product of MAO acting on a monoamine is the corresponding aldehyde, usually rapidly further oxidized by aldehyde dehydrogenase (ADH) to a carboxylic acid, which is the final excreted metabolite. Note also that the FAD-FADH2 cycle generates hydrogen peroxide which itself requires inactivation by catalase or, in the brain, glutathione peroxidase (see also Figure 7).
Figure 4
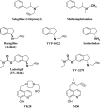
Structures of MAO inhibitors. In the top row, the structural similarity between selegiline/l-deprenyl and methamphetamine is shown. Below are the aminoindan series of propargylamine compounds such as rasagiline. Next, the bi-functional MAO and cholinesterase inhibitors (ladostigil) and lastly, the iron chelator-MAO inhibitors.
Figure 5
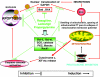
The interactions of irreversible, propargylamine-based, MAO-B inhibitors with apoptotic pathways. There are both direct effects such as inhibition of GAPDH translocation to the nucleus and indirect effects, via Bcl and Bax, etc. The overall outcome of these many interactions is a strong anti-apoptotic effect, independent of MAO inhibition.
Figure 6
Pathways of dopamine synthesis in dopaminergic neurons and metabolism by MAO-A and -B in the brain. Tyrosine passes through the blood–brain barrier and is hydroxylated by tyrosine hydroxylase (TH) to DOPA and then decarboxylated by DOPA decarboxylase (DDC) to dopamine (DA) within the neuron. Dopamine is taken up into synaptic vesicles (SV) or metabolized by MAO-A in neuronal mitochondria. After release from the terminal, extracellular dopamine is cleared by uptake into astrocytes and glia also containing MAO-A and MAO-B. Selective inhibition of one MAO isoform allows the other to metabolize dopamine effectively and does not alter the steady state levels of striatal dopamine. On the other hand, non-selective inhibition of both isoforms induces highly significant increase in striatal dopamine and in other brain regions. D1 and D2, dopamine receptors.
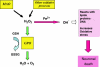
Figure 7
The mechanism of neurotoxicity induced by iron and hydrogen peroxide, via the Fenton reaction. Metabolism of monoamines by MAO is a major source of hydrogen peroxide (H2O2) in the brain. Normally the H2O2 is then inactivated by glutathione peroxidase (GPO) but it can be converted, chemically, by Fe2+ ions (Fenton reaction) into the highly reactive hydroxyl radical. This radical has widespread deleterious effects which can cause neuronal damage and death. When GSH levels are low and MAO and iron are increased (see text), the possibility of diversion of H2O2 via the Fenton reaction is correspondingly increased, with consequent increases in oxidative damage to neurons. Inhibition of MAO decreases the formation of H2O2 and iron chelation removes the Fe2+ ions, both decreasing the formation of hydroxyl radical and the levels of oxidative stress.
Similar articles
- Monoamine oxidase inhibitors, and iron chelators in depressive illness and neurodegenerative diseases.
Youdim MBH. Youdim MBH. J Neural Transm (Vienna). 2018 Nov;125(11):1719-1733. doi: 10.1007/s00702-018-1942-9. Epub 2018 Oct 19. J Neural Transm (Vienna). 2018. PMID: 30341696 Review. - Pharmacological aspects of the neuroprotective effects of irreversible MAO-B inhibitors, selegiline and rasagiline, in Parkinson's disease.
Szökő É, Tábi T, Riederer P, Vécsei L, Magyar K. Szökő É, et al. J Neural Transm (Vienna). 2018 Nov;125(11):1735-1749. doi: 10.1007/s00702-018-1853-9. Epub 2018 Feb 7. J Neural Transm (Vienna). 2018. PMID: 29417334 Review. - Selegiline: a molecule with innovative potential.
Tábi T, Vécsei L, Youdim MB, Riederer P, Szökő É. Tábi T, et al. J Neural Transm (Vienna). 2020 May;127(5):831-842. doi: 10.1007/s00702-019-02082-0. Epub 2019 Sep 27. J Neural Transm (Vienna). 2020. PMID: 31562557 Free PMC article. Review. - Neuroprotective profile of the multitarget drug rasagiline in Parkinson's disease.
Weinreb O, Amit T, Riederer P, Youdim MB, Mandel SA. Weinreb O, et al. Int Rev Neurobiol. 2011;100:127-49. doi: 10.1016/B978-0-12-386467-3.00007-8. Int Rev Neurobiol. 2011. PMID: 21971006 Review.
Cited by
- Overview of the Neuroprotective Effects of the MAO-Inhibiting Antidepressant Phenelzine.
Matveychuk D, MacKenzie EM, Kumpula D, Song MS, Holt A, Kar S, Todd KG, Wood PL, Baker GB. Matveychuk D, et al. Cell Mol Neurobiol. 2022 Jan;42(1):225-242. doi: 10.1007/s10571-021-01078-3. Epub 2021 Apr 10. Cell Mol Neurobiol. 2022. PMID: 33839994 Free PMC article. Review. - Flavonoids from Sideritis Species: Human Monoamine Oxidase (hMAO) Inhibitory Activities, Molecular Docking Studies and Crystal Structure of Xanthomicrol.
Turkmenoglu FP, Baysal İ, Ciftci-Yabanoglu S, Yelekci K, Temel H, Paşa S, Ezer N, Çalış İ, Ucar G. Turkmenoglu FP, et al. Molecules. 2015 Apr 23;20(5):7454-73. doi: 10.3390/molecules20057454. Molecules. 2015. PMID: 25915461 Free PMC article. - Essential Oils as a Potential Neuroprotective Remedy for Age-Related Neurodegenerative Diseases: A Review.
Abd Rashed A, Abd Rahman AZ, Rathi DNG. Abd Rashed A, et al. Molecules. 2021 Feb 19;26(4):1107. doi: 10.3390/molecules26041107. Molecules. 2021. PMID: 33669787 Free PMC article. Review. - Isoflavones prevent oxidative stress and inhibit the activity of the enzyme monoamine oxidase in vitro.
da Silva Schmitz I, Schaffer LF, Busanello A, de Freitas CM, Fachinetto R, Peroza LR. da Silva Schmitz I, et al. Mol Biol Rep. 2019 Apr;46(2):2285-2292. doi: 10.1007/s11033-019-04684-z. Epub 2019 Feb 12. Mol Biol Rep. 2019. PMID: 30756334 - Chronic L-dopa decreases serotonin neurons in a subregion of the dorsal raphe nucleus.
Stansley BJ, Yamamoto BK. Stansley BJ, et al. J Pharmacol Exp Ther. 2014 Nov;351(2):440-7. doi: 10.1124/jpet.114.218966. Epub 2014 Sep 11. J Pharmacol Exp Ther. 2014. PMID: 25212217 Free PMC article.
References
- BAKHLE Y.S. Pharmacokinetic and metabolic properties of lung. Br. J. Anaesth. 1990;65:79–93. - PubMed
- BIRKMAYER W., KNOLL J., RIEDERER P., YOUDIM M.B., HARS V., MARTON J. Increased life expectancy resulting from addition of L-deprenyl to Madopar treatment in Parkinson's disease: a longterm study. J. Neural. Transm. 1985;64:113–127. - PubMed
- BIRKMAYER W., RIEDERER P., YOUDIM M.B.H., LINAUER W. The potentiation of the anti-akinetic effect after L-dopa treatment by an inhibitor of MAO-B, L-deprenyl. J. Neural. Transm. 1975;36:303–326. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases