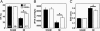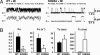Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression - PubMed (original) (raw)
Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression
Xander H T Wehrens et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Defective regulation of the cardiac ryanodine receptor (RyR2)/calcium release channel, required for excitation-contraction coupling in the heart, has been linked to cardiac arrhythmias and heart failure. For example, diastolic calcium "leak" via RyR2 channels in the sarcoplasmic reticulum has been identified as an important factor contributing to impaired contractility in heart failure and ventricular arrhythmias that cause sudden cardiac death. In patients with heart failure, chronic activation of the "fight or flight" stress response leads to protein kinase A (PKA) hyperphosphorylation of RyR2 at Ser-2808. PKA phosphorylation of RyR2 Ser-2808 reduces the binding affinity of the channel-stabilizing subunit calstabin2, resulting in leaky RyR2 channels. We developed RyR2-S2808A mice to determine whether Ser-2808 is the functional PKA phosphorylation site on RyR2. Furthermore, mice in which the RyR2 channel cannot be PKA phosphorylated were relatively protected against the development of heart failure after myocardial infarction. Taken together, these data show that PKA phosphorylation of Ser-2808 on the RyR2 channel appears to be a critical mediator of progressive cardiac dysfunction after myocardial infarction.
Figures
Fig. 1.
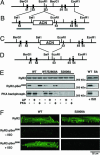
Lack of PKA phosphorylation of RyR2 in RyR2-S2808A mice. (A) The wild-type locus of the murine RyR2 gene containing exons 51–55. (B) The targeting construct containing 2.4- and 5.4-kb homologous regions (horizontal gray lines). The S2808A mutation (*) is engineered in exon 55. (C) The homologous recombinant mutant allele containing the RyR2-S2808A mutation and the ACN selection marker cassette. (D) Final RyR2-S2808A allele after excision of the ACN selection marker. (E) Representative Western blot analysis by using a phospho-specific antibody recognizing PKA-phosphorylated Ser-2808 on RyR2 and PKA kinasing reaction both demonstrate that RyR2-S2808A channels from mice cannot be PKA-phosphorylated. RyR2 immunoprecipitated from cardiac lysates from WT, heterozygous WT/RyR2-S2808A, and RyR2-S2808A mice were treated with alkaline phosphatase (AP) or protein kinase A (PKA), respectively (Left). In addition, WT and RyR2-S2808A (SA) mice were injected with isoproterenol (Right). (F) Representative confocal images of isolated cardiomyocytes immunolabeled with antibodies detecting the RyR2 protein (Top) or the PKA-phosphorylated form of RyR2 at Ser2808 (Center and Bottom). Center and Bottom represent isolated cardiomyocytes untreated or treated with isoproterenol, respectively.
Fig. 2.
PKA phosphorylation of Ser-2808 but not Ser-2031 modulates RyR2 single-channel activity. (A) Representative WT and mutant recombinant human RyR2 channels, coexpressed with calstabin2 (FKBP12.6), were PKA-phosphorylated in the presence or absence of the PKA-inhibitor PKI5–24. Alanine substitution of Ser-2808, but not Ser-2031, prevents PKA phosphorylation of RyR2. Moreover, PKA phosphorylation of WT and RyR2-S2031A channel reduced the binding affinity of the RyR2 subunit calstabin2, whereas calstabin2 did not dissociate from RyR2-S2808A channels treated with PKA. (B) Single-channel recordings of RyR2-WT and mutant RyR2. PKA phosphorylation of RyR2-WT and RyR2-S2031A channels increased Po at low-cytosolic Ca2+ concentrations (150 nM), whereas the mutant RyR2-S2808A channels did not exhibit PKA phosphorylation-induced increase in Po. The Asp substitution of Ser-2031 functionally mimicked PKA phosphorylation of this residue but did not cause an increase in Po of the RyR2-S2031D channels. In contrast, RyR2-S2808D channels mimicked constitutively PKA-phosphorylated RyR2-WT channels, confirming that Ser-2808 is the only functional PKA site on RyR2. (C) Summary values of Po of single-channel recordings described in B (n = 4–9 channels in each group).
Fig. 3.
Increased cardiac contractility in RyR2-S2808A mice 4 weeks after MI. (A) Quantification of M-mode echocardiograms showing increased ejection fraction (EF) in RyR2-S2808A mice compared with WT. *, P < 0.05. Number of mice as indicated. (B) Pressure-volume loops showing increased cardiac contractility in RyR2-S2808A mice compared with WT. dP/dt, maximum slope of the derivative of change in systolic pressure over time. (C) Echocardiographic quantification of the end-systolic diameter (ESD) showing reduced cardiac remodeling in RyR2-S2808A mice compared with WT.
Fig. 4.
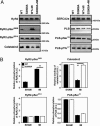
MI does not cause PKA hyperphosphorylation of RyR2 and calstabin2 dissociation in RyR2-S2808A mice. Equivalent amounts of RyR2 were immunoprecipitated from cardiac lysates by using an anti-RyR2 antibody. Representative immunoblots (A) and bar graphs (B) show the amount of PKA and CaMKII phosphorylation of RyR2 as well as the amount of calstabin2 associated with RyR2 (Left). In contrast to WT mice, RyR2-S2808A mice did not develop PKA hyperphosphorylation of RyR2 after MI. A slight reduction in SERCA2a and PLB expression was observed in both WT and S2808A mice, whereas PLB was hypophosphorylated at Ser-16 in both infarcted WT and S2808A mice (n = 9, P = N.S.).
Fig. 5.
Normalized RyR2 channel function in RyR2-S2808A mice after MI. (A) RyR2 channels isolated from hearts 28 days after MI showed reduced Po in RyR2-S2808A mice compared with WT. Representative single-channel tracings are shown at 150 nM Ca2+. Fo, frequency of channel opening (s–1); To, average open time (ms); and Tc, average closed time (ms); values correspond to the representative tracing shown. Duration upper tracings, 5 s; bottom tracings, 0.5 s. Current amplitude of full openings is 4 pA; c, closed state of channel. (B) Bar graphs show mean values for WT (n = 7 channels) and RyR2-S2808A (n = 5 channels). *, P < 0.05.
Fig. 6.
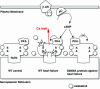
Proposed model of mechanism by which inhibition of PKA phosphorylation of RyR2 prevents intracellular Ca2+ leak in heart failure. (Left) The cardiac ryanodine receptor exists in clusters of tetrameric calcium-release channels located on the SR membrane. Each RyR2 monomer contains one PKA phosphorylation site Ser-2808 (S) and binds one PKA enzyme complex (in the cartoon, only one PKA complex is shown per tetrameric channel). (Middle) During heart failure, persistent activation of the fight-or-flight stress response causes chronic activation of the β-adrenergic signaling pathway and PKA hyperphosphorylation of RyR2 (stars depict posttranslational modification by PO4 molecules). Chronic PKA hyperphosphorylation of RyR2 is associated with calstabin2 depletion of the channel complex (symbolized by dissociation of octagons from RyR2). (Right) In RyR2-S2808A mice, substitution of Ser-2808 by Ala prevents RyR2 PKA hyperphosphorylation and SR Ca2+ leak that has beneficial effects, including reduced maladaptive remodeling after MI.
Comment in
- Profile of Andrew R. Marks.
Downey P. Downey P. Proc Natl Acad Sci U S A. 2006 Jun 13;103(24):8915-7. doi: 10.1073/pnas.0600503103. Epub 2006 Jun 5. Proc Natl Acad Sci U S A. 2006. PMID: 16754887 Free PMC article. No abstract available.
Similar articles
- Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice.
Shan J, Kushnir A, Betzenhauser MJ, Reiken S, Li J, Lehnart SE, Lindegger N, Mongillo M, Mohler PJ, Marks AR. Shan J, et al. J Clin Invest. 2010 Dec;120(12):4388-98. doi: 10.1172/JCI32726. Epub 2010 Nov 22. J Clin Invest. 2010. PMID: 21099118 Free PMC article. - Role of chronic ryanodine receptor phosphorylation in heart failure and β-adrenergic receptor blockade in mice.
Shan J, Betzenhauser MJ, Kushnir A, Reiken S, Meli AC, Wronska A, Dura M, Chen BX, Marks AR. Shan J, et al. J Clin Invest. 2010 Dec;120(12):4375-87. doi: 10.1172/JCI37649. Epub 2010 Nov 22. J Clin Invest. 2010. PMID: 21099115 Free PMC article. - Intact beta-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase A phosphorylation site in the cardiac ryanodine receptor.
Benkusky NA, Weber CS, Scherman JA, Farrell EF, Hacker TA, John MC, Powers PA, Valdivia HH. Benkusky NA, et al. Circ Res. 2007 Oct 12;101(8):819-29. doi: 10.1161/CIRCRESAHA.107.153007. Epub 2007 Aug 23. Circ Res. 2007. PMID: 17717301 - Calstabin deficiency, ryanodine receptors, and sudden cardiac death.
Lehnart SE, Wehrens XH, Marks AR. Lehnart SE, et al. Biochem Biophys Res Commun. 2004 Oct 1;322(4):1267-79. doi: 10.1016/j.bbrc.2004.08.032. Biochem Biophys Res Commun. 2004. PMID: 15336974 Review. - Ryanodine receptors, FKBP12, and heart failure.
Marks AR. Marks AR. Front Biosci. 2002 Apr 1;7:d970-7. doi: 10.2741/A822. Front Biosci. 2002. PMID: 11897558 Review.
Cited by
- Ryanodine receptor 2 inhibition reduces dispersion of cardiac repolarization, improves contractile function, and prevents sudden arrhythmic death in failing hearts.
Joshi P, Estes S, DeMazumder D, Knollmann BC, Dey S. Joshi P, et al. Elife. 2023 Dec 11;12:RP88638. doi: 10.7554/eLife.88638. Elife. 2023. PMID: 38078905 Free PMC article. Clinical Trial. - Therapeutic Approaches of Ryanodine Receptor-Associated Heart Diseases.
Szentandrássy N, Magyar ZÉ, Hevesi J, Bányász T, Nánási PP, Almássy J. Szentandrássy N, et al. Int J Mol Sci. 2022 Apr 18;23(8):4435. doi: 10.3390/ijms23084435. Int J Mol Sci. 2022. PMID: 35457253 Free PMC article. Review. - Calcium-Handling Defects and Neurodegenerative Disease.
Schrank S, Barrington N, Stutzmann GE. Schrank S, et al. Cold Spring Harb Perspect Biol. 2020 Jul 1;12(7):a035212. doi: 10.1101/cshperspect.a035212. Cold Spring Harb Perspect Biol. 2020. PMID: 31427373 Free PMC article. Review. - Calcium cycling proteins and their association with heart failure.
Hadri L, Hajjar RJ. Hadri L, et al. Clin Pharmacol Ther. 2011 Oct;90(4):620-4. doi: 10.1038/clpt.2011.161. Epub 2011 Aug 10. Clin Pharmacol Ther. 2011. PMID: 21832991 Free PMC article. Review. - Targeted ablation of cardiac sympathetic neurons reduces resting, reflex and exercise-induced sympathetic activation in conscious rats.
Lujan HL, Palani G, Chen Y, Peduzzi JD, Dicarlo SE. Lujan HL, et al. Am J Physiol Heart Circ Physiol. 2009 May;296(5):H1305-11. doi: 10.1152/ajpheart.00095.2009. Epub 2009 Mar 20. Am J Physiol Heart Circ Physiol. 2009. PMID: 19304949 Free PMC article.
References
- Wehrens, X. H. & Marks, A. R. (2004) Ann. Med. 36, Suppl. 1, 70–80. - PubMed
- Lakatta, E. G. (2004) Cell Calcium 35, 629–642. - PubMed
- Wehrens, X. H. T., Lehnart, S. E. & Marks, A. R. (2005) Annu. Rev. Physiol. 67, 69–98. - PubMed
- Song, L. S., Wang, S. Q., Xiao, R. P., Spurgeon, H., Lakatta, E. G. & Cheng, H. (2001) Circ. Res. 88, 794–801. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials