Mitochondrial DNA mutations are established in human colonic stem cells, and mutated clones expand by crypt fission - PubMed (original) (raw)
. 2006 Jan 17;103(3):714-9.
doi: 10.1073/pnas.0505903103. Epub 2006 Jan 6.
Sean L Preston, Paul J Tadrous, Robert W Taylor, Martin J Barron, Dahmane Oukrif, Simon J Leedham, Maesha Deheragoda, Peter Sasieni, Marco R Novelli, Janusz A Z Jankowski, Douglass M Turnbull, Nicholas A Wright, Stuart A C McDonald
Affiliations
- PMID: 16407113
- PMCID: PMC1325106
- DOI: 10.1073/pnas.0505903103
Mitochondrial DNA mutations are established in human colonic stem cells, and mutated clones expand by crypt fission
Laura C Greaves et al. Proc Natl Acad Sci U S A. 2006.
Abstract
The understanding of the fixation of mutations within human tissues and their subsequent clonal expansion is a considerable problem, of which little is known. We have previously shown that nononcogenic mutations in the mitochondrial genome occur in one of a number of morphologically normal colonic crypt stem cells, the progeny of which later occupy the whole crypt. We propose that these wholly mutated crypts then clonally expand by crypt fission, where each crypt divides into two mutated daughter crypts. Here we show that (i) mutated crypts in the process of fission share the same mutated mitochondrial genotype not present in neighboring cytochrome c oxidase-positive crypts (the odds of this being a random event are >or=2.48 x 10(9):1); (ii) neighboring mutated crypts have the same genotype, which is different from adjacent cytochrome c oxidase-positive crypts; (iii) mutated crypts are clustered together throughout the colon; and (iv) patches of cytochrome c oxidase-deficient crypts increase in size with age. We thus demonstrate definitively that crypt fission is the mechanism by which mutations spread in the normal human colon. This has important implications for the biology of the normal adult human colon and possibly for the growth and spread of colorectal neoplasms.
Figures
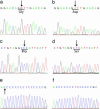
Fig. 1.
Both arms of a cytochrome c oxidase-deficient crypt in fission share identical mutations. (a–i) Serial sections were made of a single crypt in fission, and single cells were isolated from each arm and from a single neighboring cytochrome c oxidase-positive crypt. Cells from both arms of the cytochrome c oxidase-deficient crypt in fission had a common 4733 T>C transition j is the neighboring cytochrome c oxidase-positive crypt, and k is from the deficient crypt in fission.
Fig. 2.
Single cells were isolated from each of the cytochrome c oxidase-deficient crypts and from adjacent cytochrome c oxidase-positive crypts. In one patient, each cytochrome c oxidase-deficient cell had a 6277 A>G transition (a) which is not present in the cytochrome c oxidase-positive cells (b). In another patient, each cytochrome c oxidase-deficient cell had a 7275 T>C transition (c) and a C311 insertion (e) not present in the cytochrome c oxidase-positive cells (d and f).
Fig. 3.
Analysis of
clan
-generated data. (a) The observed RR for any image was >1 in all patients except patient 11 (40 years of age) who had very few mutated crypts. (b) Observed versus randomly generated data for RR, χ2, and Nprop (91.18%, 82.35%, and 88.24%, respectively) for all images achieved significance at the 95% level. (c) All images were analyzed by two independent researchers in a blinded fashion, and no intraobserver differences were found [correlation coefficient (_R_2) of 0.85].
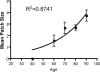
Fig. 4.
Mean patch size of cytochrome c oxidase-deficient crypts increases with age. Patients have been grouped according to decade (31–40, 41–50, 51–60, 61–70, 71–80, and 81–90). Results are ± SEM. Although individual deficient crypts were observed in patients under the age of 40, no patches of two or more deficient crypts were seen. _R_2 = 0.8741, and the equation of best-fit curve is y = 0.20090.03295x.
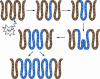
Fig. 5.
A proposed model of how DNA mutations spread in the human colon. The initial mutation can occur anywhere within the epithelium but can persist only in stem cells. Occasionally, a stem cell carrying a DNA mutation can dominate the crypt, leading to all cells within that crypt also carrying the mutation. At some point, this crypt will divide by fission, and this process will carry on through the life of the host. If the mutation is prooncogenic, this may affect the rate at which this occurs, resulting in a rapid spread of potentially cancerous mutations.
Similar articles
- Clonal expansion in the human gut: mitochondrial DNA mutations show us the way.
McDonald SA, Preston SL, Greaves LC, Leedham SJ, Lovell MA, Jankowski JA, Turnbull DM, Wright NA. McDonald SA, et al. Cell Cycle. 2006 Apr;5(8):808-11. doi: 10.4161/cc.5.8.2641. Epub 2006 Apr 17. Cell Cycle. 2006. PMID: 16628008 Review. - Analysis of the clonal architecture of the human small intestinal epithelium establishes a common stem cell for all lineages and reveals a mechanism for the fixation and spread of mutations.
Gutierrez-Gonzalez L, Deheragoda M, Elia G, Leedham SJ, Shankar A, Imber C, Jankowski JA, Turnbull DM, Novelli M, Wright NA, McDonald SA. Gutierrez-Gonzalez L, et al. J Pathol. 2009 Mar;217(4):489-96. doi: 10.1002/path.2502. J Pathol. 2009. PMID: 19156773 - Use of methylation patterns to determine expansion of stem cell clones in human colon tissue.
Graham TA, Humphries A, Sanders T, Rodriguez-Justo M, Tadrous PJ, Preston SL, Novelli MR, Leedham SJ, McDonald SA, Wright NA. Graham TA, et al. Gastroenterology. 2011 Apr;140(4):1241-1250.e1-9. doi: 10.1053/j.gastro.2010.12.036. Epub 2010 Dec 28. Gastroenterology. 2011. PMID: 21192938 - A Diffusion-like Process Accommodates New Crypts During Clonal Expansion in Human Colonic Epithelium.
Olpe C, Khamis D, Chukanova M, Skoufou-Papoutsaki N, Kemp R, Marks K, Tatton C, Lindskog C, Nicholson A, Brunton-Sim R, Malhotra S, Ten Hoopen R, Stanley R, Winton DJ, Morrissey E. Olpe C, et al. Gastroenterology. 2021 Aug;161(2):548-559.e23. doi: 10.1053/j.gastro.2021.04.035. Epub 2021 Apr 22. Gastroenterology. 2021. PMID: 33895166 Free PMC article. - Measuring stem cell dynamics in the human colon--where there's a wiggle, there's a way.
Leedham SJ. Leedham SJ. J Pathol. 2014 Nov;234(3):292-5. doi: 10.1002/path.4422. J Pathol. 2014. PMID: 25112223 Free PMC article. Review.
Cited by
- Paneth Cell-Rich Regions Separated by a Cluster of Lgr5+ Cells Initiate Crypt Fission in the Intestinal Stem Cell Niche.
Langlands AJ, Almet AA, Appleton PL, Newton IP, Osborne JM, Näthke IS. Langlands AJ, et al. PLoS Biol. 2016 Jun 27;14(6):e1002491. doi: 10.1371/journal.pbio.1002491. eCollection 2016 Jun. PLoS Biol. 2016. PMID: 27348469 Free PMC article. - Clonal expansions in ulcerative colitis identify patients with neoplasia.
Salk JJ, Salipante SJ, Risques RA, Crispin DA, Li L, Bronner MP, Brentnall TA, Rabinovitch PS, Horwitz MS, Loeb LA. Salk JJ, et al. Proc Natl Acad Sci U S A. 2009 Dec 8;106(49):20871-6. doi: 10.1073/pnas.0909428106. Epub 2009 Nov 19. Proc Natl Acad Sci U S A. 2009. PMID: 19926851 Free PMC article. - Field defects in progression to gastrointestinal tract cancers.
Bernstein C, Bernstein H, Payne CM, Dvorak K, Garewal H. Bernstein C, et al. Cancer Lett. 2008 Feb 18;260(1-2):1-10. doi: 10.1016/j.canlet.2007.11.027. Epub 2007 Dec 31. Cancer Lett. 2008. PMID: 18164807 Free PMC article. Review. - Fluctuating methylation clocks for cell lineage tracing at high temporal resolution in human tissues.
Gabbutt C, Schenck RO, Weisenberger DJ, Kimberley C, Berner A, Househam J, Lakatos E, Robertson-Tessi M, Martin I, Patel R, Clark SK, Latchford A, Barnes CP, Leedham SJ, Anderson ARA, Graham TA, Shibata D. Gabbutt C, et al. Nat Biotechnol. 2022 May;40(5):720-730. doi: 10.1038/s41587-021-01109-w. Epub 2022 Jan 3. Nat Biotechnol. 2022. PMID: 34980912 Free PMC article. - Predominant Asymmetrical Stem Cell Fate Outcome Limits the Rate of Niche Succession in Human Colonic Crypts.
Stamp C, Zupanic A, Sachdeva A, Stoll EA, Shanley DP, Mathers JC, Kirkwood TBL, Heer R, Simons BD, Turnbull DM, Greaves LC. Stamp C, et al. EBioMedicine. 2018 May;31:166-173. doi: 10.1016/j.ebiom.2018.04.017. Epub 2018 Apr 25. EBioMedicine. 2018. PMID: 29748033 Free PMC article. Clinical Trial.
References
- Karam, S. M. (1999) Front. Biosci. 4, D286–D298. - PubMed
- Wright, N. A. & Alison, M. R. (1984) The Biology of Epithelial Cell Populations (Clarendon, Oxford, U.K.).
- Preston, S. L., Wong, W. M., Chan, A. O., Poulsom, R., Jeffery, R., Goodlad, R. A., Mandir, N., Elia, G., Novelli, M., Bodmer, W. F., et al. (2003) Cancer Res. 63, 3819–3825. - PubMed
- Brierley, E. J., Johnson, M. A., Lightowlers, R. N., James, O. F. & Turnbull, D. M. (1998) Ann. Neurol. 43, 217–223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases