A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis - PubMed (original) (raw)
Case Reports
A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis
Melanie J Percy et al. Proc Natl Acad Sci U S A. 2006.
Abstract
The number of red blood cells is normally tightly regulated by a classic homeostatic mechanism based on oxygen sensing in the kidney. Decreased oxygen delivery resulting from anemia induces the production of erythropoietin, which increases red cell production and hence oxygen delivery. Investigations of erythropoietin regulation identified the transcription factor hypoxia-inducible factor (HIF). HIF is now recognized as being a key regulator of genes that function in a comprehensive range of processes besides erythropoiesis, including energy metabolism and angiogenesis. HIF itself is regulated through the alpha-subunit, which is hydroxylated in the presence of oxygen by a family of three prolyl hydroxylase domain proteins (PHDs)/HIF prolyl hydroxylases/egg-laying-defective nine enzymes. Hydroxylation allows capture by the von Hippel-Lindau tumor suppressor gene product, ubiquitination, and destruction by the proteasome. Here we describe an inherited mutation in a mammalian PHD enzyme. We show that this mutation in PHD2 results in a marked decrease in enzyme activity and is associated with familial erythrocytosis, identifying a previously unrecognized cause of this condition. Our findings indicate that PHD2 is critical for normal regulation of HIF in humans.
Figures
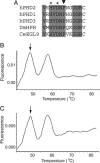
Fig. 1.
The 950 C → G mutation affects a residue in the active site of PHD2. (A) Comparison of amino acid sequence from residues 311–323 (human PHD2 numbering) in human HIF prolyl hydroxylases as well as those from Drosophila melanogaster (DmHPH) and Caenorhabditis elegans (CeEGL9). Shading indicates completely conserved residues, asterisks indicate iron-chelating residues, and the inverted triangle indicates Pro-317 of human PHD2, which is predicted to be changed to Arg by the 950 C → G mutation. (B and C) Detection of 950 C → G PHD2 mutation by a hybridization probe. By using a fluorescently labeled hybridization probe specific to the normal sequence, the two heterozygous erythrocytosis siblings were screened with the Roche Diagnostics LightCycler. The annealing temperature of the probe was 58°C when hybridized to the normal sequence. The mismatch due to the presence of the 950 C → G PHD2 mutation reduced the annealing temperature to 49°C (arrows). Both siblings exhibited two peaks, indicating the presence of both the normal and mutant sequences.
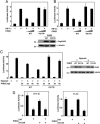
Fig. 2.
PHD2 P317R is defective in HIF binding and HIF hydroxylase activity. (A) 35S-labeled, _in vitro_-translated wild-type or P317R Flag-PHD2 was incubated with 40 μg of either GST or GST-HIF-1α-(549–575) immobilized on GSH-agarose. The agarose was washed, bound proteins were eluted, and the eluates were subjected to SDS/PAGE and autoradiography. Input represented 10% of the total. The relative recovery of wild-type PHD2 from three replicates was 100 ± 8.7 (arbitrary units), whereas that of P317R PHD2 was 2.5 ± 1.3 (P < 0.001). (B) 35S-labeled, _in vitro_-translated wild-type or P317R Flag-PHD2 was incubated with 60 μg of either GST or GST-HIF-2α-(516–550) immobilized on GSH-agarose. The agarose was washed, the bound proteins were eluted, and the eluates were subjected to SDS/PAGE and autoradiography. Input represented 10% of the total. The relative recovery of wild-type PHD2 from three replicates was 100 ± 19 (arbitrary units), whereas that of P317R PHD2 was 4.2 ± 0.42 (P = 0.001). (C) _In vitro_-translated wild-type or P317R Flag-PHD2, or mock in vitro translation reaction, was incubated with 20 μg of GST-HIF-1α-(549–575) immobilized on 10 μl of GSH-agarose in the presence of 2-oxoglutarate, ascorbic acid, and FeCl2. The agarose was then washed, and the degree of HIF hydroxylation was assessed by subsequent incubation with 35S-labeled, _in vitro_-translated VHL. Input represented 5% of the total. Under the conditions of the assay, the recovery of 35S-labeled, _in vitro_-translated VHL when wild-type PHD2 was used in three independent experiments was 100 ± 10.2 (arbitrary units), whereas that when P317R PHD2 was used was 8.8 ± 3.4 (P < 0.001).
Fig. 3.
P317R PHD2 is defective in inhibiting HRE reporter gene activity. (A) HEK293 cells were cotransfected with 100 ng of (eHRE)3-Luc, which contains the Epo HRE, 100 ng of pRL-TK, 200 ng of either pcDNA3 or pcDNA3-HA-HIF-1α, and 0, 5, or 15 ng of pcDNA3-Flag-PHD2 (wild type or P317R). The total DNA dose was adjusted to 415 ng with pcDNA3. Eighteen hours after transfection, the cells were harvested and assayed for luciferase activity. Error bars represent standard deviations. In separate experiments, HEK293 cells were transfected with 1 μg of wild-type or P317R pcDNA3-Flag-PHD2, and 18 h later, 20-μg extracts were analyzed by Western blotting with anti-Flag or anti-β-tubulin antibodies. The positions of PHD2, β-tubulin, and a molecular weight marker are indicated. (B) HEK293 cells were cotransfected with 100 ng of (eHRE)3-Luc, 100 ng of pRL-TK, 300 ng of either pcDNA3 or pSV-Sport-HA-hHIF-2α, and 0, 2, or 6 ng of pcDNA3-Flag-PHD2 (wild type or P317R). The total DNA dose was adjusted to 506 ng with pcDNA3. Eighteen hours after transfection, the cells were harvested and assayed for luciferase activity. (C) HEK293 cells were cotransfected with 50 ng of (eHRE)3-Luc, 100 ng of pRL-TK, and either 0, 20, or 60 ng of pcDNA3-Flag-PHD2 (wild type or P317R). The total DNA dose was adjusted to 210 ng with pcDNA3. Twenty-four hours after transfection, some cells were subjected to 1% O2 for an additional 18 h. All cells were then harvested and assayed for luciferase activity. In separate experiments, HEK293 cells were transfected with 0.5 μg of wild-type or P317R pcDNA3-Flag-PHD2, and 24 h later, some cells were subjected to 1% O2 for an additional 18 h. Cellular extracts (10 μg) were analyzed by Western blotting with anti-Flag or anti-β-tubulin antibodies. (D) HEK293 cells were cotransfected with 50 ng of (eHRE)3-Luc, 100 ng of pRL-TK, and either 0 or 100 ng of wild-type or P317R pcDNA3-Flag-PHD2. The total DNA dose was equalized with pcDNA3. (Left) Cells were harvested 42 h after transfection and assayed for luciferase activity. (Right) Results from a similar experiment, except that at 24 h after transfection, cells were subjected to 1% O2 for 18 h. With all luciferase assays, activities are normalized to that of a Renilla luciferase internal transfection control. Shown are results that are representative of two or three independent experiments performed in duplicate.
Similar articles
- Oxygen sensing: recent insights from idiopathic erythrocytosis.
Lee FS, Percy MJ, McMullin MF. Lee FS, et al. Cell Cycle. 2006 May;5(9):941-5. doi: 10.4161/cc.5.9.2723. Epub 2006 May 1. Cell Cycle. 2006. PMID: 16687917 Free PMC article. - A novel erythrocytosis-associated PHD2 mutation suggests the location of a HIF binding groove.
Percy MJ, Furlow PW, Beer PA, Lappin TR, McMullin MF, Lee FS. Percy MJ, et al. Blood. 2007 Sep 15;110(6):2193-6. doi: 10.1182/blood-2007-04-084434. Epub 2007 Jun 19. Blood. 2007. PMID: 17579185 Free PMC article. - Involvement of oxygen-sensing pathways in physiologic and pathologic erythropoiesis.
Semenza GL. Semenza GL. Blood. 2009 Sep 3;114(10):2015-9. doi: 10.1182/blood-2009-05-189985. Epub 2009 Jun 3. Blood. 2009. PMID: 19494350 Review. - The HIF pathway and erythrocytosis.
Lee FS, Percy MJ. Lee FS, et al. Annu Rev Pathol. 2011;6:165-92. doi: 10.1146/annurev-pathol-011110-130321. Annu Rev Pathol. 2011. PMID: 20939709 Review. - Erythrocytosis-associated HIF-2alpha mutations demonstrate a critical role for residues C-terminal to the hydroxylacceptor proline.
Furlow PW, Percy MJ, Sutherland S, Bierl C, McMullin MF, Master SR, Lappin TR, Lee FS. Furlow PW, et al. J Biol Chem. 2009 Apr 3;284(14):9050-8. doi: 10.1074/jbc.M808737200. Epub 2009 Feb 10. J Biol Chem. 2009. PMID: 19208626 Free PMC article.
Cited by
- Hypoxia Signaling Cascade for Erythropoietin Production in Hepatocytes.
Tojo Y, Sekine H, Hirano I, Pan X, Souma T, Tsujita T, Kawaguchi S, Takeda N, Takeda K, Fong GH, Dan T, Ichinose M, Miyata T, Yamamoto M, Suzuki N. Tojo Y, et al. Mol Cell Biol. 2015 Aug;35(15):2658-72. doi: 10.1128/MCB.00161-15. Epub 2015 May 26. Mol Cell Biol. 2015. PMID: 26012551 Free PMC article. - Genetic modifications of EGLN1 reactivate HbF production in β0-thalassemia/HbE.
Jan-Ngam V, Boontha S, Tubsuwan A, Wongpalee SP, Fanhchaksai K, Tantiworawit A, Charoenkwan P, Khamphikham P. Jan-Ngam V, et al. Heliyon. 2024 Sep 18;10(18):e38020. doi: 10.1016/j.heliyon.2024.e38020. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39381253 Free PMC article. - Overexpression of HIF prolyl-hydoxylase-2 transgene in the renal medulla induced a salt sensitive hypertension.
Zhu Q, Liu M, Han WQ, Li PL, Wang Z, Li N. Zhu Q, et al. J Cell Mol Med. 2012 Nov;16(11):2701-7. doi: 10.1111/j.1582-4934.2012.01590.x. J Cell Mol Med. 2012. PMID: 22686466 Free PMC article. - Low oxygen stimulates the intellect. Symposium on hypoxia and development, physiology and disease.
Koumenis C, Maxwell PH. Koumenis C, et al. EMBO Rep. 2006 Jul;7(7):679-84. doi: 10.1038/sj.embor.7400733. EMBO Rep. 2006. PMID: 16799463 Free PMC article. No abstract available. - Genetic causes of erythrocytosis and the oxygen-sensing pathway.
Lee FS. Lee FS. Blood Rev. 2008 Nov;22(6):321-32. doi: 10.1016/j.blre.2008.04.003. Epub 2008 Jun 5. Blood Rev. 2008. PMID: 18538455 Free PMC article. Review.
References
- Ivan, M., Kondo, K., Yang, H., Kim, W., Valiando, J., Ohh, M., Salic, A., Asara, J. M., Lane, W. S. & Kaelin, W. G., Jr. (2001) Science 292, 464–468. - PubMed
- Jaakkola, P., Mole, D. R., Tian, Y. M., Wilson, M. I., Gielbert, J., Gaskell, S. J., Kriegsheim Av, A., Hebestreit, H. F., Mukherji, M., Schofield, C. J., et al. (2001) Science 292, 468–472. - PubMed
- Epstein, A. C., Gleadle, J. M., McNeill, L. A., Hewitson, K. S., O'Rourke, J., Mole, D. R., Mukherji, M., Metzen, E., Wilson, M. I., Dhanda, A., et al. (2001) Cell 107, 43–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases