Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice - PubMed (original) (raw)
Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice
Melinda C Myzak et al. FASEB J. 2006 Mar.
Abstract
Sulforaphane (SFN) is an isothiocyanate from broccoli that induces phase 2 detoxification enzymes. We recently reported that SFN acts as a histone deacetylase (HDAC) inhibitor in human colon cancer cells in vitro, and the present study sought to extend these findings in vivo. In mice treated with a single oral dose of 10 mumol SFN, there was significant inhibition of HDAC activity in the colonic mucosa after 6 h, and immunoblots revealed a concomitant increase in acetylated histones H3 and H4, which returned to control levels by 48 h. Longer-term treatment with SFN in the diet resulted in levels of acetylated histones and p21(WAF1) in the ileum, colon, prostate, and peripheral blood mononuclear cells that were elevated compared with controls. Consistent with these findings, SFN suppressed tumor development in Apc(min) mice, and there was an increase in acetylated histones in the polyps, including acetylated histones specifically associated with the promoter region of the P21 and bax genes. These results provide the first evidence for HDAC inhibition by SFN in vivo and imply that such a mechanism might contribute to the cancer chemoprotective and therapeutic effects of SFN, alone or in combination with other HDAC inhibitors currently undergoing clinical trials.
Figures
Figure 1
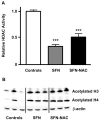
SFN and SFN-NAC inhibit HDAC activity and induce accumulation of acetylated histones in mouse colonic mucosa. Mice were treated by gavage with 10 μmol SFN, 10 μmol SFN-NAC, or vehicle alone, and colonic mucosa was isolated after 6 h. A) HDAC activities, expressed as mean ± SE, n = 3; ***P < 0.001. B) Colonic mucosa from each mouse was analyzed by immunoblot for acetylated histone H3 and acetylated histone H4; β-actin was used as a loading control.
Figure 2
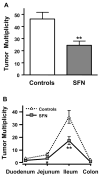
Dietary SFN suppresses polyp formation in _Apc_min mice. Mice consumed ~6 μmol SFN/day for 10 wk in the diet or AIN93G diet alone (controls). A) Suppression of overall tumor multiplicity; B) Suppression according to tumor distribution in the GI tract. Mean ± SE, n = 15; *P < 0.05; **P < 0.01.
Figure 3
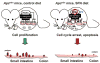
Summary of key findings. In the _Apc_min mouse, HDAC/co-repressor complexes maintain a tightly restricted chromatin configuration, which limits access of transcription factors to DNA, and represses genes required for cell cycle checkpoint control and apoptosis. Inhibition of HDAC by SFN enables acetyl groups (“ac”) to be added to histone tails, loosening DNA/chromatin interactions, and allowing access of transcription factors to the promoters of genes such as P21 and bax. Re-expression of these genes triggers cell cycle arrest and apoptosis in transformed cells and microadenomas, thereby suppressing polyp formation.
Similar articles
- A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase.
Myzak MC, Karplus PA, Chung FL, Dashwood RH. Myzak MC, et al. Cancer Res. 2004 Aug 15;64(16):5767-74. doi: 10.1158/0008-5472.CAN-04-1326. Cancer Res. 2004. PMID: 15313918 - Dietary histone deacetylase inhibitors: from cells to mice to man.
Dashwood RH, Ho E. Dashwood RH, et al. Semin Cancer Biol. 2007 Oct;17(5):363-9. doi: 10.1016/j.semcancer.2007.04.001. Epub 2007 May 5. Semin Cancer Biol. 2007. PMID: 17555985 Free PMC article. Review. - Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells.
Clarke JD, Hsu A, Yu Z, Dashwood RH, Ho E. Clarke JD, et al. Mol Nutr Food Res. 2011 Jul;55(7):999-1009. doi: 10.1002/mnfr.201000547. Epub 2011 Mar 4. Mol Nutr Food Res. 2011. PMID: 21374800 Free PMC article. - Sulforaphane suppresses in vitro and in vivo lung tumorigenesis through downregulation of HDAC activity.
Jiang LL, Zhou SJ, Zhang XM, Chen HQ, Liu W. Jiang LL, et al. Biomed Pharmacother. 2016 Mar;78:74-80. doi: 10.1016/j.biopha.2015.11.007. Epub 2016 Jan 14. Biomed Pharmacother. 2016. PMID: 26898427 - Modulation of histone deacetylase activity by dietary isothiocyanates and allyl sulfides: studies with sulforaphane and garlic organosulfur compounds.
Nian H, Delage B, Ho E, Dashwood RH. Nian H, et al. Environ Mol Mutagen. 2009 Apr;50(3):213-21. doi: 10.1002/em.20454. Environ Mol Mutagen. 2009. PMID: 19197985 Free PMC article. Review.
Cited by
- Sulforaphane recovers cone function in an Nrf2-dependent manner in middle-aged mice undergoing RPE oxidative stress.
Qi X, Walton DA, Plafker KS, Boulton ME, Plafker SM. Qi X, et al. Mol Vis. 2022 Oct 16;28:378-393. eCollection 2022. Mol Vis. 2022. PMID: 36338670 Free PMC article. - Apoptosis evasion via long non-coding RNAs in colorectal cancer.
Irfan M, Javed Z, Khan K, Khan N, Docea AO, Calina D, Sharifi-Rad J, Cho WC. Irfan M, et al. Cancer Cell Int. 2022 Sep 8;22(1):280. doi: 10.1186/s12935-022-02695-8. Cancer Cell Int. 2022. PMID: 36076273 Free PMC article. Review. - Impact of Epigenetic Dietary Components on Cancer through Histone Modifications.
Gao Y, Tollefsbol TO. Gao Y, et al. Curr Med Chem. 2015;22(17):2051-64. doi: 10.2174/0929867322666150420102641. Curr Med Chem. 2015. PMID: 25891109 Free PMC article. Review. - Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells.
Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E. Myzak MC, et al. Carcinogenesis. 2006 Apr;27(4):811-9. doi: 10.1093/carcin/bgi265. Epub 2005 Nov 9. Carcinogenesis. 2006. PMID: 16280330 Free PMC article. - Sulforaphane absorption and histone deacetylase activity following single dosing of broccoli sprout supplement in normal dogs.
Curran KM, Bracha S, Wong CP, Beaver LM, Stevens JF, Ho E. Curran KM, et al. Vet Med Sci. 2018 Nov;4(4):357-363. doi: 10.1002/vms3.118. Epub 2018 Aug 17. Vet Med Sci. 2018. PMID: 30117668 Free PMC article.
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA090890/CA/NCI NIH HHS/United States
- P01 CA090890-01A20003/CA/NCI NIH HHS/United States
- P30 ES00210/ES/NIEHS NIH HHS/United States
- CA-65525/CA/NCI NIH HHS/United States
- R01 CA080176/CA/NCI NIH HHS/United States
- CA-107693/CA/NCI NIH HHS/United States
- P01 CA090890-05S1/CA/NCI NIH HHS/United States
- CA-100608/CA/NCI NIH HHS/United States
- CA-80176/CA/NCI NIH HHS/United States
- P01 CA090890-05/CA/NCI NIH HHS/United States
- R01 CA065525/CA/NCI NIH HHS/United States
- R01 CA065525-08/CA/NCI NIH HHS/United States
- R01 CA065525-09/CA/NCI NIH HHS/United States
- R01 CA080176-05/CA/NCI NIH HHS/United States
- CA-90890/CA/NCI NIH HHS/United States
- R01 CA080176-04/CA/NCI NIH HHS/United States
- P01 CA090890-01A29001/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous