Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis - PubMed (original) (raw)
Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis
Kazuya Tsujita et al. J Cell Biol. 2006.
Abstract
The conserved FER-CIP4 homology (FCH) domain is found in the pombe Cdc15 homology (PCH) protein family members, including formin-binding protein 17 (FBP17). However, the amino acid sequence homology extends beyond the FCH domain. We have termed this region the extended FC (EFC) domain. We found that FBP17 coordinated membrane deformation with actin cytoskeleton reorganization during endocytosis. The EFC domains of FBP17, CIP4, and other PCH protein family members show weak homology to the Bin-amphiphysin-Rvs (BAR) domain. The EFC domains bound strongly to phosphatidylserine and phosphatidylinositol 4,5-bisphosphate and deformed the plasma membrane and liposomes into narrow tubules. Most PCH proteins possess an SH3 domain that is known to bind to dynamin and that recruited and activated neural Wiskott-Aldrich syndrome protein (N-WASP) at the plasma membrane. FBP17 and/or CIP4 contributed to the formation of the protein complex, including N-WASP and dynamin-2, in the early stage of endocytosis. Furthermore, knockdown of endogenous FBP17 and CIP4 impaired endocytosis. Our data indicate that PCH protein family members couple membrane deformation to actin cytoskeleton reorganization in various cellular processes.
Figures
Figure 1.
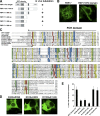
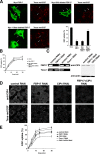
Identification of functional EFC domain. (A) Domain structures of FBP17 and deletion constructs. CC, coiled–coil; P, proline-rich motif. Tubulation ability is also indicated. (B) COS-7 cells were transfected with GFP-tagged FBP17 or GFP-tagged FBP17 1–300 aa, and GFP-fluorescence was observed by confocal microscopy. Bar, 20 μm. (C) Sequence alignment of the EFC domain. This alignment was obtained by Clustal W (Thompson et al., 1997). Conserved residues are highlighted with the following color code: yellow, hydrophobic; green, polar; blue, basic; red, acidic. Asterisks show the mutation sites. (D) Effect of the GFP-tagged K33Q + R35Q, K51Q + K52Q, R113Q + K114Q and mutants of the EFC domain of FBP17 on tubulation in COS-7 cells. Bar, 20 μm. (E) 50 cells overexpressing GFP-tagged K33Q + R35Q, K51Q + K52Q, R113Q + K114Q, K138Q + K139Q, and K171Q + R173Q mutants scored for tabulation. the results are presented as percentages. Three independent experiments were performed. All error bars indicate SEM.
Figure 2.
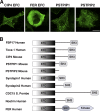
EFC domain in other proteins. (A) In vivo tubulation by overexpression of the GFP-tagged CIP4 EFC domain, GFP-tagged FER EFC domain, GFP-tagged PSTPIP1, and GFP-tagged PSTPIP2 in COS-7 cells. Bar, 20 μm. (B) The domain organization of the EFC domain–containing protein.
Figure 3.
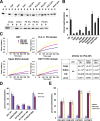
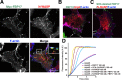
The EFC is a novel phosphoinositide binding module. (A) The EFC domain of FBP17 strongly bound to PS and PI(4,5)P2. (top) Brain lipid (Folch lipid) or synthetic PE/PC liposomes supplemented with 10% of the indicated lipid were incubated with the EFC domain and sedimented, and then stained with Coomassie brilliant blue. (bottom) Synthetic PE/PC liposomes supplemented with various percentages of PS were also analyzed. S, supernatant; P, pellet. (B) Quantitative representation of A. Three independent experiments were performed and the protein band intensity was measured. (C) The dissociation constant (K d) of the indicated domain for PI(4,5)P2 was determined using dual polarization interferometer. The response curves (left) used for the calculation of K d (right) were shown. (D) Comparison of the lipid binding of wild type and mutants of the FBP17 EFC domain examined by sedimentation assay. Three independent experiments were performed. (E) Sedimentation assays with the EFC domain of CIP4 and FER, or PSTPIP1 and PSTPIP2, to examine the association with lipids. Three independent experiments were performed. All error bars indicate SEM.
Figure 4.
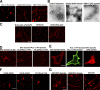
The EFC domain alone tubulates protein-free liposomes in vitro. (A) Brain lipid liposomes containing 5% rhodamine-conjugated PE (rhodamine-PE) were incubated with the PLCδ1 PH domain, amphiphysin2/Bin 1 BAR domain, or FBP17 EFC domain and examined by confocal microscopy. Bar, 5 μm. (B) Brain lipid liposomes were incubated with Amph2 BAR domain or FBP17 EFC domain and examined by electron microscopy. Bar, 200 nm. (C) Brain lipid liposomes containing 5% rhodamine-PE and 10% PI(4,5)P2 or synthetic lipid liposomes containing 70% PE, 15% PC, 5% rhodamine-PE, and either 10% PI, PI(4,5)P2, or PI(3,4,5)P3 were incubated with the EFC domain. Afterward, the liposomes were examined by confocal microscopy. Bar, 10 μm. (D) 3 μM of the EFC domain was incubated with brain lipid liposomes containing 5% of rhodamine-PE and 10% of PI(4,5)P2 as in C (left, EFC domain). The PLCδ1 PH domain (3 or 9 μM) was preincubated with these liposomes for 2 min, and then the liposomes were incubated with 3 μM of the EFC domain for 2 min and examined by confocal microscopy. (E) HA-tagged FBP17 EFC domain alone or with GFP-tagged PLCδ1 PH domain (green) was transfected into COS-7 cells. The EFC domain protein was visualized with anti-HA antibody (red). Bar, 20 μm. (F) Brain liposomes containing 5% rhodamine-PE and 10% PI(4,5)P2 were incubated with the K33Q + R35Q, K51Q + K52Q, and R113Q + K114Q mutant. Bar, 5 μm. (G) Brain lipid liposomes containing 5% of rhodamine-PE and 10% of PI(4,5)P2 were incubated with the EFC domain of CIP4, FER, or PSTPIP1. Bar, 5 μm.
Figure 5.
FBP17 is required for endocytosis of EGFR. (A) Myc-tagged wild-type FBP17, SH3 domain–deleted FBP17, and 1–56 aa–deleted FBP17 were transfected in COS-7 cells. After starvation for 16 h, the transfected cells were incubated with Texas red–EGF (red) for 15 min, fixed, and stained with anti-Myc antibody (green). The percentage of cells with internalized EGF among FBP17-overexpressing cells was also shown with SD. Bar, 20 μm. (B) Quantitative EGF internalization assay of FBP17-transfected cells. The histogram shows uptake of biotinylated EGF as a function of total bound biotinylated EGF at indicated times in transfected cells. Data from three independent experiments. Error bars represent SD. (C) A431cells were transfected with the control, FBP17, CIP4, and Toca-1siRNA. After 24 h, a second transfection was performed and the cells were cultured for an additional 72 h and subjected to RT-PCR and Western blotting. (D) After 96 h, the cells transfected with the siRNA were incubated with Texas red-EGF for 10 min, fixed, and stained with anti-CIP4 antibody. (E) Quantitative EGF internalization assay of siRNA-transfected cells as in B. Data from three independent experiments. All error bars indicate SEM.
Figure 6.
FBP17 recruits N-WASP to plasma membrane where it activates actin polymerization. (A) Myc-tagged FBP17 was transfected in COS-7 cells. The cells were stained with anti-Myc antibody (green) and anti–N-WASP antibody (red), and incubated with Alexa Fluor 647–conjugated phalloidin (blue) for visualization of actin filaments. Cells with expression of FBP17 at a low level is shown. Bar, 20 μm. (B) Myc-tagged FBP17 transfected in COS-7 cells were stained with anti-Myc antibody (green) and anti-Arp2 antibody (red) and incubated with Alexa Fluor 647–conjugated phalloidin (blue). (C) Myc-tagged SH3 domain–deleted FBP17 was transfected into COS-7 cells and the cells were stained by the same method as in A. (D) In vitro actin polymerization assay was performed with 0.2 μM of pyrene-labeled monomeric actin, 2 μM of unlabeled monomeric actin, 60 nM Arp2/3 complex, and 100 nM verprolin homology/cofilin homology/acidic region of N-WASP or 100 nM N-WASP, with or without GST-tagged FBP17 at various concentrations.
Figure 7.
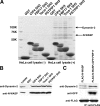
The PCH proteins bind to N-WASP and dynamin-2 via the SH3 domain. (A) The pull-down assay was shown. GST alone, or GST-CIP4 SH3 domain, GST-FBP17 SH3 domain, GST-Toca-1 SH3 domain, and PSTPIP1 SH3 domain were immobilized on glutathione–sepharose beads and incubated with HeLa cell lysate. After washing, each sample was subjected to SDS-PAGE analysis and stained with Coomassie brilliant blue. (B) Pull-down samples were analyzed by Western blotting with anti–Dynamin-2 antibody or anti–N-WASP antibody. (C) Immunoprecipitation analysis. The cells transfected with FLAG-tagged N-WASP and/or GFP tagged FBP17 were immunoprecipitated with anti-FLAG. Coprecipitation of Dynamin-2 was analyzed by Western blotting.
Figure 8.
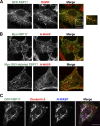
EGFR, dynamin-2, and N-WASP are associated with invaginating tubules induced by FBP17. (A) GFP-FBP17 was transfected in COS-7 cells. After starvation for 16 h, the transfected cells were stimulated with 100 ng/ml EGF for 15 min, fixed, and stained by anti-GFP (green) and anti-EGFR (red) antibodies. (B) Myc-tagged FBP17 and SH3 domain–deleted FBP17-transfected COS-7 cells were stained with anti-Myc (green) and anti–N-WASP (red) antibodies. Bar, 20 μm. (C) GFP-tagged FBP17 was transfected in COS-7 cells and stained with anti–dynamin-2 (red) and anti–N-WASP (blue) antibodies. Bar, 20 μm.
Figure 9.
Actin polymerization and formation of FBP17-associated tubules. (A) Myc-tagged FBP17 alone or Myc-tagged FBP17 plus Flag-tagged N-WASP were transfected in COS-7 cells. The cells were stained with anti-Myc (green) antibody, anti–N-WASP antibody (red), and Alexa Fluor 647–conjugated phalloidin (blue). Bar, 20 μm. (B) Quantitative analysis of plasma membrane tubulation upon N-WASP overexpression. n = 50 cells. Three independent experiments were performed. Error bars represent SD. (C) GFP-FBP17 transfected COS-7 cells were treated with DMSO or latrunculin B (LatB; 5 μM) for 5 min. Bar, 20 μm. (D) Quantitative analysis of induction of tubular structures after latrunculin B treatment. n = 50 cells. Three independent experiments were performed. All error bars indicate SEM.
Similar articles
- EFC/F-BAR proteins and the N-WASP-WIP complex induce membrane curvature-dependent actin polymerization.
Takano K, Toyooka K, Suetsugu S. Takano K, et al. EMBO J. 2008 Nov 5;27(21):2817-28. doi: 10.1038/emboj.2008.216. Epub 2008 Oct 16. EMBO J. 2008. PMID: 18923421 Free PMC article. - Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins.
Itoh T, Erdmann KS, Roux A, Habermann B, Werner H, De Camilli P. Itoh T, et al. Dev Cell. 2005 Dec;9(6):791-804. doi: 10.1016/j.devcel.2005.11.005. Dev Cell. 2005. PMID: 16326391 - Pombe Cdc15 homology (PCH) proteins: coordinators of membrane-cytoskeletal interactions.
Chitu V, Stanley ER. Chitu V, et al. Trends Cell Biol. 2007 Mar;17(3):145-56. doi: 10.1016/j.tcb.2007.01.003. Epub 2007 Feb 12. Trends Cell Biol. 2007. PMID: 17296299 Review. - Syndapin--a membrane remodelling and endocytic F-BAR protein.
Quan A, Robinson PJ. Quan A, et al. FEBS J. 2013 Nov;280(21):5198-212. doi: 10.1111/febs.12343. Epub 2013 Jul 5. FEBS J. 2013. PMID: 23668323 Review.
Cited by
- Calcium oscillations-coupled conversion of actin travelling waves to standing oscillations.
Wu M, Wu X, De Camilli P. Wu M, et al. Proc Natl Acad Sci U S A. 2013 Jan 22;110(4):1339-44. doi: 10.1073/pnas.1221538110. Epub 2013 Jan 7. Proc Natl Acad Sci U S A. 2013. PMID: 23297209 Free PMC article. - Fer kinase limits neutrophil chemotaxis toward end target chemoattractants.
Khajah M, Andonegui G, Chan R, Craig AW, Greer PA, McCafferty DM. Khajah M, et al. J Immunol. 2013 Mar 1;190(5):2208-16. doi: 10.4049/jimmunol.1200322. Epub 2013 Jan 25. J Immunol. 2013. PMID: 23355730 Free PMC article. - The state of F-BAR domains as membrane-bound oligomeric platforms.
Snider CE, Wan Mohamad Noor WNI, Nguyen NTH, Gould KL, Suetsugu S. Snider CE, et al. Trends Cell Biol. 2021 Aug;31(8):644-655. doi: 10.1016/j.tcb.2021.03.013. Epub 2021 Apr 20. Trends Cell Biol. 2021. PMID: 33888395 Free PMC article. Review. - Through its F-BAR and RhoGAP domains, Rgd1p acts in different polarized growth processes in budding yeast.
Lefebvre F, Prouzet-Mauléon V, Vieillemard A, Thoraval D, Crouzet M, Doignon F. Lefebvre F, et al. Commun Integr Biol. 2009;2(2):120-2. doi: 10.4161/cib.7737. Commun Integr Biol. 2009. PMID: 19704907 Free PMC article. - The intrinsically disordered region of the cytokinetic F-BAR protein Cdc15 performs a unique essential function in maintenance of cytokinetic ring integrity.
Mangione MC, Snider CE, Gould KL. Mangione MC, et al. Mol Biol Cell. 2019 Oct 15;30(22):2790-2801. doi: 10.1091/mbc.E19-06-0314. Epub 2019 Sep 11. Mol Biol Cell. 2019. PMID: 31509478 Free PMC article.
References
- Aspenstrom, P. 1997. A Cdc42 target protein with homology to the non-kinase domain of FER has a potential role in regulating the actin cytoskeleton. Curr. Biol. 7:479–487. - PubMed
- Engqvist-Goldstein, A.E., and D.G. Drubin. 2003. Actin assembly and endocytosis: from yeast to mammals. Annu. Rev. Cell Dev. Biol. 19:287–332. - PubMed
- Fankhauser, C., A. Reymond, L. Cerutti, S. Utzig, K. Hofmann, and V. Simanis. 1995. The S. pombe cdc15 gene is a key element in the reorganization of F-actin at mitosis. Cell. 82:435–444. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases