The telomeric PARP, tankyrases, as targets for cancer therapy - PubMed (original) (raw)
Review
The telomeric PARP, tankyrases, as targets for cancer therapy
H Seimiya. Br J Cancer. 2006.
Abstract
The requirement for the maintenance of telomeres by telomerase by most cancer cells for continued proliferation is a target in anticancer strategies. Tankyrases are poly(ADP-ribose) polymerases that enhance telomerase access to telomeres. Tankyrase 1 modulates telomerase inhibition in human cancer cells and is reviewed in this report as a potential telomere-directed anticancer target.
Figures
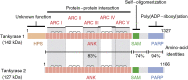
Figure 1
Structures of tankyrase 1 and tankyrase 2. HPS, homopolymeric runs of His, Pro, and Ser, without known functions; ANK, ankyrin domain, consisting of 24 ANK repeats; SAM, multimerization domain homologous to the sterile alpha motif; PARP, PARP catalytic domain that adds ADP-ribose chains onto acceptor proteins. The ANK domain is further divided into five well-conserved ANK repeat clusters (ARC), each of which contributes to ligand binding. Bridges above two adjacent ANK repeats indicate the presence of a conserved histidine contributing to inter-repeat stabilization.
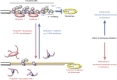
Figure 2
Telomere elongation by tankyrase 1 and impact on telomerase inhibitors. For telomere elongation, active telomerase needs to gain access to the telomeric 3′-overhang. The TRF1-TIN2-TPP1-POT1 telomeric protein complex limits telomerase access, whereas tankyrase 1 removes the telomeric protein complex by poly(ADP-ribosyl)ating TRF1. Either telomere shortening or tankyrase 1 upregulation, each of which decreases the TRF1-TIN2-TPP1-POT1 loading on a chromosome end, attenuates the impact of telomerase inhibitors by allowing access of residual telomerase activity. Conversely, blockade of tankyrase 1 enhances the effect of telomerase inhibitors. The relative importance of tankyrase 1 vs tankyrase 2 inhibition remains unclear.
Similar articles
- Tankyrase 1 as a target for telomere-directed molecular cancer therapeutics.
Seimiya H, Muramatsu Y, Ohishi T, Tsuruo T. Seimiya H, et al. Cancer Cell. 2005 Jan;7(1):25-37. doi: 10.1016/j.ccr.2004.11.021. Cancer Cell. 2005. PMID: 15652747 - Evaluation of tankyrase inhibition in whole cells.
Ohishi T, Tsuruo T, Seimiya H. Ohishi T, et al. Methods Mol Biol. 2007;405:133-46. doi: 10.1007/978-1-60327-070-0_11. Methods Mol Biol. 2007. PMID: 18369822 - Mechanism-based combination telomerase inhibition therapy.
Shay JW, Wright WE. Shay JW, et al. Cancer Cell. 2005 Jan;7(1):1-2. doi: 10.1016/j.ccr.2004.12.012. Cancer Cell. 2005. PMID: 15652743 - Telomerase inhibitors in cancer therapy: current status and future directions.
Incles CM, Schultes CM, Neidle S. Incles CM, et al. Curr Opin Investig Drugs. 2003 Jun;4(6):675-85. Curr Opin Investig Drugs. 2003. PMID: 12901225 Review. - Telomere maintenance mechanisms as a target for drug development.
Bearss DJ, Hurley LH, Von Hoff DD. Bearss DJ, et al. Oncogene. 2000 Dec 27;19(56):6632-41. doi: 10.1038/sj.onc.1204092. Oncogene. 2000. PMID: 11426649 Review.
Cited by
- Tankyrase and the canonical Wnt pathway protect lung cancer cells from EGFR inhibition.
Casás-Selves M, Kim J, Zhang Z, Helfrich BA, Gao D, Porter CC, Scarborough HA, Bunn PA Jr, Chan DC, Tan AC, DeGregori J. Casás-Selves M, et al. Cancer Res. 2012 Aug 15;72(16):4154-64. doi: 10.1158/0008-5472.CAN-11-2848. Epub 2012 Jun 27. Cancer Res. 2012. PMID: 22738915 Free PMC article. - RDX induces aberrant expression of microRNAs in mouse brain and liver.
Zhang B, Pan X. Zhang B, et al. Environ Health Perspect. 2009 Feb;117(2):231-40. doi: 10.1289/ehp.11841. Epub 2008 Sep 19. Environ Health Perspect. 2009. PMID: 19270793 Free PMC article. - RK-287107, a potent and specific tankyrase inhibitor, blocks colorectal cancer cell growth in a preclinical model.
Mizutani A, Yashiroda Y, Muramatsu Y, Yoshida H, Chikada T, Tsumura T, Okue M, Shirai F, Fukami T, Yoshida M, Seimiya H. Mizutani A, et al. Cancer Sci. 2018 Dec;109(12):4003-4014. doi: 10.1111/cas.13805. Epub 2018 Oct 20. Cancer Sci. 2018. PMID: 30238564 Free PMC article. - Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases.
Morales J, Li L, Fattah FJ, Dong Y, Bey EA, Patel M, Gao J, Boothman DA. Morales J, et al. Crit Rev Eukaryot Gene Expr. 2014;24(1):15-28. doi: 10.1615/critreveukaryotgeneexpr.2013006875. Crit Rev Eukaryot Gene Expr. 2014. PMID: 24579667 Free PMC article. Review. - The PARP family: insights into functional aspects of poly (ADP-ribose) polymerase-1 in cell growth and survival.
Jubin T, Kadam A, Jariwala M, Bhatt S, Sutariya S, Gani AR, Gautam S, Begum R. Jubin T, et al. Cell Prolif. 2016 Aug;49(4):421-37. doi: 10.1111/cpr.12268. Epub 2016 Jun 22. Cell Prolif. 2016. PMID: 27329285 Free PMC article. Review.
References
- Bae J, Donigian JR, Hsueh AJ (2003) Tankyrase 1 interacts with Mcl-1 proteins and inhibits their regulation of apoptosis. J Biol Chem 278: 5195–5204 - PubMed
- Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T (2005) Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434: 913–917 - PubMed
- Chang P, Coughlin M, Mitchison TJ (2005a) Tankyrase-1 polymerization of poly(ADP-ribose) is required for spindle structure and function. Nat Cell Biol 7: 1133–1139 - PubMed
- Chang P, Jacobson MK, Mitchison TJ (2004) Poly(ADP-ribose) is required for spindle assembly and structure. Nature 432: 645–649 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources