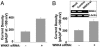Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms - PubMed (original) (raw)
Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms
Ahmed Lazrak et al. Proc Natl Acad Sci U S A. 2006.
Abstract
WNK kinases are serine-threonine kinases with an atypical placement of the catalytic lysine. Intronic deletions with increased expression of a ubiquitous long WNK1 transcript cause pseudohypoaldosteronism type 2 (PHA II), characterized by hypertension and hyperkalemia. Here, we report that long WNK1 inhibited ROMK1 by stimulating its endocytosis. Inhibition of ROMK by long WNK1 was synergistic with, but not dependent on, WNK4. A smaller transcript of WNK1 lacking the N-terminal 1-437 amino acids is expressed highly in the kidney. Whether expression of the KS-WNK1 (kidney-specific, KS) is altered in PHA II is not known. We found that KS-WNK1 did not inhibit ROMK1 but reversed the inhibition of ROMK1 caused by long WNK1. Consistent with the lack of inhibition by KS-WNK1, we found that amino acids 1-491 of the long WNK1 were sufficient for inhibiting ROMK. Dietary K(+) restriction decreases ROMK abundance in the renal cortical-collecting ducts by stimulating endocytosis, an adaptative response important for conservation of K(+) during K(+) deficiency. We found that K(+) restriction in rats increased whole-kidney transcript of long WNK1 while decreasing that of KS-WNK1. Thus, KS-WNK1 is a physiological antagonist of long WNK1. Hyperkalemia in PHA II patients with PHA II mutations may be caused, at least partially, by increased expression of long WNK1 with or without decreased expression of KS-WNK1.
Figures
Fig. 1.
Effect of WNK1 on ROMK1 expressed in HEK cells. (A) Whole-cell recording, voltage-clamp protocol, and current-voltage (I-V) relationships of currents. (B) Dose-dependent inhibition of ROMK1 by WNK1. Cells were transfected with ROMK1 plus WNK1 (0-2.5 μg of plasmid DNA). In each experiment, the total amount of DNA for transfection was balanced by using empty vector. Ba2+-sensitive (after subtraction of residual currents in the presence of 10 mM Ba2+) inward current density is shown. *, P < 0.05 vs. ROMK1 alone. (C) Effect of wild-type (WT-DII) or dominant-negative (K44A) dynamin II (DN-DII) on WNK1 inhibition of ROMK1. (D) Effect of WNK1 on wild-type ROMK1 (WT-RK) vs. N375I ROMK1 mutant. Experiments above were repeated 3-5 times with similar results. NS, not significant.
Fig. 2.
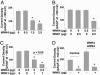
Effect of WNK1 or WNK4 siRNA on ROMK1 expression in HEK cells. (A) Cells were cotransfected with ROMK1 (0.5 μg) plus WNK1 siRNA (200 nM in transfection mixture) or control oligonucleotide. (B) Cells were cotransfected with ROMK1 (0.5 μg) plus WNK4 siRNA (200 nM) or control oligonucleotide. (B Inset) mRNA expression of endogenous WNK4 analyzed by reversetranscription PCR. Cells were mock-transfected (Mock) or transfected with control oligos (Control) or siRNA for WNK4 (WNK4-si). Experiments above were repeated 2-3 times with similar results.
Fig. 3.
Relationships between WNK1 and WNK4 regulation of ROMK1. (A) Dose-dependent inhibition of ROMK1 by WNK4. Cells were transfected with ROMK1 plus WNK4 (0-2.5 μg plasmid DNA). (B) Cells were cotransfected with ROMK1 plus 0.5 μg WNK1 and/or 0.5 μg WNK4. (C) Cells were cotransfected with ROMK1 plus 1 μg WNK1 and/or 0.5 μg WNK4. (D) Cells were cotransfected with ROMK1 (0.5 μg), WNK1 (2.5 μg), and WNK4 siRNA (200 nM) or control oligonucleotide. *, P < 0.05 vs. ROMK1 alone. Experiments above were repeated 3-5 times with similar results.
Fig. 4.
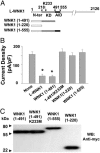
Domain of WNK1 involved in regulation of ROMK1. (A) Domain structure of full-length long WNK1 and location of the catalytic lysine-233. Autoinhibitory domain (AID), kinase domain (KD), and N terminus preceding kinase domain (N-ter) are shown, but not drawn in scale. Fragments of WNK1 used are shown. (B) Cells were transfected with ROMK1 alone or cotransfected with indicated Myc-tagged WNK1 construct (each at 0.5 μg). Experiments above were repeated three times with similar results. *, P < 0.05 vs. ROMK1 alone. (C) Western blot analysis of each construct blotted by anti-Myc antibody. Arrowhead on the left indicates molecular mass in kilodaltons (kDa). Experiments above were repeated three times with similar results.
Fig. 5.
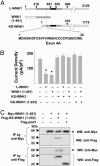
Role of KS-WNK1 in regulation of ROMK1. (A) Comparison of domain structure of long WNK1 (L-WNK1) with KS-WNK1. KS-WNK1 lacks the first 437 amino acids of L-WNK1 but contains unique 30 amino acids coded by an alternatively spliced exon 4A (the first exon of KS-WNK1). The vertical dotted line indicates position of amino acid in L-WNK1 equivalent to amino acid 31 of KS-WNK1. Amino acids of L-WNK1 and KS-WNK1 distal to the dotted line are identical. Amino acid 660 of L-WNK1 is equal to amino acid 253 of KS-WNK1. (B) Cells with transfected with ROMK alone or cotransfected with ROMK1 plus L-WNK1, WNK1 (1-491), and/or KS-WNK1 or KS-WNK1 (1-253) as indicated. (C) Lysates from mock-transfected cells or cells cotransfected with Myc-tagged WNK1 (1-491), Flag-tagged KS-WNK1 (1-253), and/or an unrelated Flag-tagged protein pod1 were immunoprecipitated by either anti-Myc or anti-Flag antibody and probed for Western blot analysis by the respective antibody as indicated. The molecular mass of Myc-WNK1 (1-491), Flag-KS-WNK1 (1-253), and Flag-pod1 are 60, 32, and 22 kDa, respectively (as indicated by arrowhead). Experiments above were repeated three times with similar results.
Fig. 6.
Effect of dietary K+ intake on long and KS-WNK1 expression in kidney. (A) Relative abundance of transcript (normalized to the control K+ diet) for long WNK1 in rat kidney fed low (LK), control (CK) or high K+ diet (HK). (B) Relative abundance for KS-WNK1. (C) Ratio of abundance of transcript for L-WNK1 vs. for KS-WNK1. Ratio relative to the control K+ diet is shown. *, P < 0.05 vs. CK.
Similar articles
- Regulation of ROMK channel and K+ homeostasis by kidney-specific WNK1 kinase.
Liu Z, Wang HR, Huang CL. Liu Z, et al. J Biol Chem. 2009 May 1;284(18):12198-206. doi: 10.1074/jbc.M806551200. Epub 2009 Feb 25. J Biol Chem. 2009. PMID: 19244242 Free PMC article. - Intersectin links WNK kinases to endocytosis of ROMK1.
He G, Wang HR, Huang SK, Huang CL. He G, et al. J Clin Invest. 2007 Apr;117(4):1078-87. doi: 10.1172/JCI30087. Epub 2007 Mar 22. J Clin Invest. 2007. PMID: 17380208 Free PMC article. - Role of WNK4 and kidney-specific WNK1 in mediating the effect of high dietary K+ intake on ROMK channel in the distal convoluted tubule.
Wu P, Gao ZX, Su XT, Ellison DH, Hadchouel J, Teulon J, Wang WH. Wu P, et al. Am J Physiol Renal Physiol. 2018 Aug 1;315(2):F223-F230. doi: 10.1152/ajprenal.00050.2018. Epub 2018 Apr 18. Am J Physiol Renal Physiol. 2018. PMID: 29667910 Free PMC article. - Mechanisms of type I and type II pseudohypoaldosteronism.
Furgeson SB, Linas S. Furgeson SB, et al. J Am Soc Nephrol. 2010 Nov;21(11):1842-5. doi: 10.1681/ASN.2010050457. Epub 2010 Sep 9. J Am Soc Nephrol. 2010. PMID: 20829405 Review. - Role of with-no-lysine [K] kinases in the pathogenesis of Gordon's syndrome.
Xie J, Craig L, Cobb MH, Huang CL. Xie J, et al. Pediatr Nephrol. 2006 Sep;21(9):1231-6. doi: 10.1007/s00467-006-0106-6. Epub 2006 May 9. Pediatr Nephrol. 2006. PMID: 16683163 Review.
Cited by
- Association of urinary sodium/potassium ratio with blood pressure: sex and racial differences.
Hedayati SS, Minhajuddin AT, Ijaz A, Moe OW, Elsayed EF, Reilly RF, Huang CL. Hedayati SS, et al. Clin J Am Soc Nephrol. 2012 Feb;7(2):315-22. doi: 10.2215/CJN.02060311. Epub 2011 Nov 23. Clin J Am Soc Nephrol. 2012. PMID: 22114147 Free PMC article. - WNK1 kinase isoform switch regulates renal potassium excretion.
Wade JB, Fang L, Liu J, Li D, Yang CL, Subramanya AR, Maouyo D, Mason A, Ellison DH, Welling PA. Wade JB, et al. Proc Natl Acad Sci U S A. 2006 May 30;103(22):8558-63. doi: 10.1073/pnas.0603109103. Epub 2006 May 18. Proc Natl Acad Sci U S A. 2006. PMID: 16709664 Free PMC article. - Multigene kinase network, kidney transport, and salt in essential hypertension.
Welling PA, Chang YP, Delpire E, Wade JB. Welling PA, et al. Kidney Int. 2010 Jun;77(12):1063-9. doi: 10.1038/ki.2010.103. Epub 2010 Apr 14. Kidney Int. 2010. PMID: 20375989 Free PMC article. Review. - Pathophysiological roles of WNK kinases in the kidney.
Uchida S. Uchida S. Pflugers Arch. 2010 Sep;460(4):695-702. doi: 10.1007/s00424-010-0848-7. Epub 2010 May 21. Pflugers Arch. 2010. PMID: 20490538 Review. - Regulation of ROMK channel and K+ homeostasis by kidney-specific WNK1 kinase.
Liu Z, Wang HR, Huang CL. Liu Z, et al. J Biol Chem. 2009 May 1;284(18):12198-206. doi: 10.1074/jbc.M806551200. Epub 2009 Feb 25. J Biol Chem. 2009. PMID: 19244242 Free PMC article.
References
- Verissimo, F. & Jordan, P. (2001) Oncogene 20, 5562-5569. - PubMed
- Xu, B., English, J. M., Wilsbacher, J. L., Stippec, S., Goldsmith, E. J. & Cobb, M. H. (2000) J. Biol. Chem. 275, 16795-16801. - PubMed
- Min, X., Lee, B. H., Cobb, M. H. & Goldsmith, E. J. (2004) Structure (London) 12, 1303-1311. - PubMed
- Xu, B., Min, X., Stippec, S., Lee, B. H., Goldsmith, E. J. & Cobb, M. H. (2002) J. Biol. Chem. 277, 48456-48462. - PubMed
- Gordon, R. D. (1986) Hypertension 8, 93-102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK054368/DK/NIDDK NIH HHS/United States
- DK 59530/DK/NIDDK NIH HHS/United States
- DK 59530-S1/DK/NIDDK NIH HHS/United States
- DK 54368/DK/NIDDK NIH HHS/United States
- R01 DK059530/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical