Receptor activator of NF-kappaB ligand regulates the proliferation of mammary epithelial cells via Id2 - PubMed (original) (raw)
Receptor activator of NF-kappaB ligand regulates the proliferation of mammary epithelial cells via Id2
Nam-Shik Kim et al. Mol Cell Biol. 2006 Feb.
Abstract
Receptor activator of NF-kappaB ligand (RANKL) is a key regulator for mammary gland development during pregnancy. RANKL-deficient mice display impaired development of lobulo-alveolar mammary structures. Similar mammary gland defects have been reported in mice lacking Id2. Here we report that RANKL induces the proliferation of mammary epithelial cells via Id2. RANKL triggers marked nuclear translocation of Id2 in mammary epithelial cells. In vivo studies further demonstrated the defective nuclear translocation of Id2, but the normal expression of cyclin D1, in the mammary epithelial cells of rankl-/- mice. In vitro studies with nuclear localization sequence-tagged Id2 revealed that the nuclear localization of Id2 itself is critical for the downregulation of p21 promoter activity. Moreover, RANKL stimulation failed to induce cell growth and to downregulate p21 expression in Id2-/- mammary epithelial cells. Our results indicate that the inhibitor of helix-loop-helix protein, Id2, is critical to control the proliferation of mammary epithelial cells in response to RANKL stimulation.
Figures
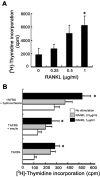
FIG. 1.
RANKL induces the proliferation of primary mammary epithelial cells. (A) Serum-starved primary mammary epithelial cells, isolated from 14.5-day pregnant mice, were stimulated with the indicated doses of RANKL in DMEM containing 10% FBS for 24 h. Cells were incubated with 1 μCi of [3H]thymidine/ml for the last 12 h of culture, and [3H]thymidine incorporation was measured. ✽, significant difference (P < 0.001). (B) Primary mammary epithelial cells isolated from 14.5-day pregnant mice were stimulated with the indicated doses of RANKL in DMEM containing 1% FBS and growth factors for 60 h. Cells were incubated with 1 μCi of [3H]thymidine/ml for the last 24 h of culture, and [3H]thymidine incorporation was measured. The results are shown as mean values ± the standard error of the mean of three separate experiments. ✽, significant difference (P < 0.01).
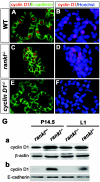
FIG. 2.
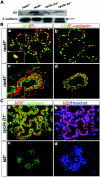
Cyclin D1 expression in rankl −/− mammary glands. (A to F) Immunohistochemistry of cyclin D1 in wild-type (A and B) and rankl−/− (C and D) mammary glands. Tissue sections of mammary glands at 1 day of lactation (L1) from the specified genotypes were stained with anti-cyclin D1 (red)/anti-E-cadherin (green) antibodies (A, C, and E) and Hoechst (B, D, and F). The specificity of antibody staining for cyclin D1 was confirmed by its absence in cyclin D1 −/− mammary epithelium (E and F). (G) Western blot analysis of lysates from rankl+/− and _rankl_−/− mammary tissues at P14.5 and L1. Actin (a) and E-cadherin (b) were used as loading controls. Similar results were obtained from three independent experiments.
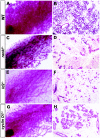
FIG. 3.
The mammary gland defects in rankl −/− mice resemble those of Id2 −/− mice but not cyclin D1 −/− mice. Whole-mount carmine-alum strain (A, C, E, and G) and histological (B, D, F, and H) analysis by hematoxylin-eosin staining of no. 4 abdominal glands from wild-type (WT) (A and B), rankl −/− (C and D), Id2 −/− (E and F), and cyclin D1 −/− (G and H) mice at L1. Note the substantial alveolar formation in cyclin D1 −/− mice compared to those in rankl −/− and Id2 −/− mice.
FIG. 4.
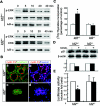
RANKL induces the nuclear translocation of Id2 in HC11 and primary mammary epithelial cells. (A) Primary MECs from P14.5 mice were treated with 1 μg of RANKL/ml for the indicated times. Cytoplasmic (C) and nuclear (N) proteins were analyzed by Western blotting for the presence of Id2. Protein disulfide isomerase (PDI) and poly(ADP-ribose) polymerase (PARP) are shown as loading controls for cytoplasmic and nuclear proteins, respectively. One result, representative of four independent experiments, is shown. (B) Primary MECs from P14.5 mice were placed in DMEM containing 1% FBS for 24 h. Serum-starved cells were untreated (left panels) or stimulated (right panels) with 1 μg of RANKL/ml for 3 h. Id2 was detected with an anti-Id2 antibody, followed by Alexa 594-labeled anti-rabbit IgG antibody (red; upper panel). Nuclear DNA was stained with Hoechst (blue; middle panel). Lower panels indicate merged images. At the bottom, the numbers indicate the localization of Id2 in the cytoplasm (C) and nucleus (N) of each cell. The values shown are means ± the standard error of the mean of three separate experiments. One result, representative of three independent experiments, is shown. (C) HC11 cells transiently transfected with HA-tagged Id2 were placed in RPMI containing 0.5% FBS for 24 h. Serum-starved cells were untreated (left panels) or stimulated (right panels) with 1 μg of RANKL/ml for 3 h. HA epitopes were detected with an anti-HA antibody, followed by Alexa 594-labeled anti-mouse IgG antibody (red; upper panels). Nuclear DNA was stained with Hoechst (blue; middle panels). Lower panels indicate merged images. One result, representative of four independent experiments, is shown.
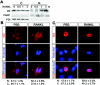
FIG.5.
Impaired nuclear translocation of Id2 in _rankl_−/− mammary glands. (A) Western blot analysis of Id2 in rankl+/−, _rankl_−/−, cyclin D1+/− and _cyclin D1_−/− mammary glands at L1. E-cadherin is shown as a protein loading control (black arrow). (B) Localization of Id2 in rankl+/− (upper panels) and _rankl_−/− (lower panels) mammary gland epithelial cells. Tissue sections of mammary glands from rankl+/− and _rankl_−/− mice at L1 were stained with anti-Id2 (a to d; green) and anti-E-cadherin (b and d; red) antibodies. Nuclear DNA was stained with PI (a and c; red). Images were acquired by using a confocal microscope (Leica Dmire2). Note the complete absence of Id2 in the nuclei of _rankl_−/− epithelial cells. Magnification, ×400. The magnified images are shown in the insets. (C) Tissue sections of mammary glands from _cyclin D1_−/− (upper panels) and _Id2_−/− (lower panels) mice at L1 were stained with anti-Id2 (a to d; red) and anti-E-cadherin (a and c; green) antibodies. Nuclear DNA was stained with Hoechst (b and d; blue). The specificity of antibody staining for Id2 was confirmed by its absence in _Id2_−/− mammary epithelium (c and d).
FIG. 6.
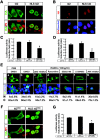
Defective RANKL-mediated downregulation of p21 expression and proliferation in Id2−/− mammary epithelial cells. (A) Activation of ERK and IκB in P14.5 Id2 +/− and Id2 −/− mammary epithelial cells after RANKL treatment. Cells were treated with 1 μg of RANKL/ml for the indicated times. The phosphorylated form of ERK (p-ERK) in whole-cell extracts was detected with the phospho-specific antibody. The membrane was stripped and probed with antibodies against ERK and IκB-α as indicated. One representative of three independent experiments is shown. (B) Immunohistochemistry of cyclin D1 from Id2 +/− (a and b) and _Id2_−/− (c and d) mice. Tissue sections of mammary glands from Id2+/− and _Id2_−/− mice at L1 were stained with anti-cyclin D1 (red)/anti-E-cadherin (green) antibodies (a and c) and Hoechst (b and d; blue). (C) Proliferation of mammary epithelial cells. Primary mammary epithelial cells from P14.5 Id2 +/− and Id2 −/− mice were stimulated with the indicated doses of RANKL for 24 h. Cells were incubated with 1 μCi of [3H]thymidine/ml for the last 12 h of culture, and [3H]thymidine incorporation was measured. The results are shown as mean values ± the standard error of the mean of three separate experiments. ✽, significant difference (P < 0.001). (D) Western blotting of p21 expression in Id2 −/− primary mammary epithelial cells after RANKL stimulation. Serum-starved primary mammary epithelial cells from P14.5 Id2 +/− and Id2 −/− mice were stimulated without (−) or with (+) 1 μg of RANKL/ml for 12 h. Actin is shown as a protein loading control. Relative expression levels of p21 to actin are indicated in the bar graphs. One result, representative of four independent experiments, is shown. (E) p21 promoter luciferase assays in primary mammary epithelial cells from P14.5 pregnant mice. Mammary epithelial cells transfected with p21-luciferase were left untreated (□) or treated with 1 μg of RANKL/ml (▪) for 24 h. Luciferase reporter activity was normalized to Renilla luciferase activity. The results are shown as mean values ± the standard error of the mean of five separate transfection experiments. ✽, significant difference (P < 0.001).
FIG. 7.
Nuclear localization of Id2 in mammary epithelial cells. (A and B) Localization and p21 promoter luciferase assays of Id2 and NLS-tagged Id2. MCF7 cells (A) and primary mammary epithelial cells from rankl −/− P14.5 mice (B) were transiently transfected with HA-Id2 (Id2) and NLS-HA-Id2 (NLS-Id2) constructs and placed in DMEM containing 10% FBS for 48 h. HA epitopes were detected with an anti-HA antibody, followed by Alexa 488-labeled anti-mouse IgG antibody (A, green) and Alexa 594-labeled anti-mouse IgG antibody (B, red). Nuclear DNA was stained with PI (A, red) and Hoechst (B, blue). One result, representative of three independent experiments, is shown. (C and D) p21 promoter luciferase assays in MCF7 cells (C) and primary mammary epithelial cells from rankl −/− P14.5 mice (D). Cells were cotransfected with p21-luciferase and Id2 constructs and, after 48 h, the luciferase reporter activity was measured and normalized to the Renilla luciferase activity. The results are shown as mean values ± the standard error of the mean of three separate transfection experiments. ✽, significant difference (P < 0.001). (E) Localization of Id2 in MCF7 cells after RANKL treatment in the presence of kinase inhibitors. MCF7 cells transiently transfected with the HA-Id2 construct were placed in DMEM containing 1% FBS for 12 h. Serum-starved cells were treated with dimethyl sulfoxide (a and b) or the indicated kinase inhibitors (c to g) for 3 h. Inhibitor-pretreated cells were untreated (a) or stimulated with 1 μg of RANKL/ml for 3 h (b to g). HA epitopes were detected with an anti-HA antibody, followed by Alexa 488-labeled anti-mouse IgG antibody (green). Nuclear DNA was stained with Hoechst (blue). One result, representative of two independent experiments, is shown. (F and G) Localization (F) and p21 promoter luciferase activity (G) of Id2S5A and NLS-Id2S5A in MCF7 cells. These experiments were performed as described for panels A and C. One result, representative of two independent experiments, is shown. Magnification, ×400.
Similar articles
- RANK overexpression in transgenic mice with mouse mammary tumor virus promoter-controlled RANK increases proliferation and impairs alveolar differentiation in the mammary epithelia and disrupts lumen formation in cultured epithelial acini.
Gonzalez-Suarez E, Branstetter D, Armstrong A, Dinh H, Blumberg H, Dougall WC. Gonzalez-Suarez E, et al. Mol Cell Biol. 2007 Feb;27(4):1442-54. doi: 10.1128/MCB.01298-06. Epub 2006 Dec 4. Mol Cell Biol. 2007. PMID: 17145767 Free PMC article. - Survival and differentiation of mammary epithelial cells in mammary gland development require nuclear retention of Id2 due to RANK signaling.
Kim NS, Kim HT, Kwon MC, Choi SW, Kim YY, Yoon KJ, Koo BK, Kong MP, Shin J, Cho Y, Kong YY. Kim NS, et al. Mol Cell Biol. 2011 Dec;31(23):4775-88. doi: 10.1128/MCB.05646-11. Epub 2011 Sep 26. Mol Cell Biol. 2011. PMID: 21947283 Free PMC article. - Regulation of Id2 expression by CCAAT/enhancer binding protein beta.
Karaya K, Mori S, Kimoto H, Shima Y, Tsuji Y, Kurooka H, Akira S, Yokota Y. Karaya K, et al. Nucleic Acids Res. 2005 Apr 4;33(6):1924-34. doi: 10.1093/nar/gki339. Print 2005. Nucleic Acids Res. 2005. PMID: 15809228 Free PMC article. - In vivo function of a differentiation inhibitor, Id2.
Yokota Y, Mori S, Narumi O, Kitajima K. Yokota Y, et al. IUBMB Life. 2001 Apr;51(4):207-14. doi: 10.1080/152165401753311744. IUBMB Life. 2001. PMID: 11569914 Review. - [Receptor activator of NF-kappaB ligand and mammary development].
Meng FX, Zang XY. Meng FX, et al. Sheng Li Ke Xue Jin Zhan. 2004 Oct;35(4):355-7. Sheng Li Ke Xue Jin Zhan. 2004. PMID: 15727219 Review. Chinese. No abstract available.
Cited by
- Microarray and pathway analysis of two COMMA-Dβ derived clones reveal important differences relevant to their developmental capacity in-vivo.
Johnson JR, Boulanger CA, Hudson T, Savage E, Smith GH. Johnson JR, et al. Oncotarget. 2019 Mar 15;10(22):2118-2135. doi: 10.18632/oncotarget.26655. eCollection 2019 Mar 15. Oncotarget. 2019. PMID: 31040905 Free PMC article. - Parathyroid Hormone-Related Protein (PTHrP) Accelerates Soluble RANKL Signals for Downregulation of Osteogenesis of Bone Mesenchymal Stem Cells.
Elango J, Rahman SU, Henrotin Y, de Val JEMS, Bao B, Wang S, Li B, Wu W. Elango J, et al. J Clin Med. 2019 Jun 12;8(6):836. doi: 10.3390/jcm8060836. J Clin Med. 2019. PMID: 31212822 Free PMC article. - RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling.
Tobeiha M, Moghadasian MH, Amin N, Jafarnejad S. Tobeiha M, et al. Biomed Res Int. 2020 Feb 19;2020:6910312. doi: 10.1155/2020/6910312. eCollection 2020. Biomed Res Int. 2020. PMID: 32149122 Free PMC article. Review. - Progesterone receptor isoform functions in normal breast development and breast cancer.
Kariagina A, Aupperlee MD, Haslam SZ. Kariagina A, et al. Crit Rev Eukaryot Gene Expr. 2008;18(1):11-33. doi: 10.1615/critreveukargeneexpr.v18.i1.20. Crit Rev Eukaryot Gene Expr. 2008. PMID: 18197783 Free PMC article. Review. - Systemic and Local Strategies for Primary Prevention of Breast Cancer.
Zaluzec EK, Sempere LF. Zaluzec EK, et al. Cancers (Basel). 2024 Jan 5;16(2):248. doi: 10.3390/cancers16020248. Cancers (Basel). 2024. PMID: 38254741 Free PMC article. Review.
References
- Anderson, D. M., E. Maraskovsky, W. L. Billingsley, W. C. Dougall, M. E. Tometsko, E. R. Roux, M. C. Teepe, R. F. DuBose, D. Cosman, and L. Galibert. 1997. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390:175-179. - PubMed
- Brisken, C., A. Ayyannan, C. Nguyen, A. Heineman, F. Reinhardt, J. Tan, S. K. Dey, G. P. Dotto, R. A. Weinberg, and T. Jan. 2002. IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Dev. Cell 3:877-887. - PubMed
- Brisken, C., S. Kaur, T. E. Chavarria, N. Binart, R. L. Sutherland, R. A. Weinberg, P. A. Kelly, and C. J. Ormandy. 1999. Prolactin controls mammary gland development via direct and indirect mechanisms. Dev. Biol. 210:96-106. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials