The Bordetella bronchiseptica type III secretion system inhibits gamma interferon production that is required for efficient antibody-mediated bacterial clearance - PubMed (original) (raw)
The Bordetella bronchiseptica type III secretion system inhibits gamma interferon production that is required for efficient antibody-mediated bacterial clearance
Mylisa R Pilione et al. Infect Immun. 2006 Feb.
Abstract
Several species of pathogenic microorganisms have developed strategies to survive and persist in vital organs which are normally maintained as sterile by the generation of strong immune responses. Here, we report an immunomodulation involving the Bordetella bronchiseptica type III secretion system (TTSS) which contributes to bacterial survival in the lower respiratory tract of the host. The prolonged persistence of B. bronchiseptica that was observed in gamma interferon (IFN-gamma)-/- mice indicates that the efficient clearance of bacteria from the lower respiratory tract requires not only B cells and antibodies but also IFN-gamma production. Our data also suggest that interleukin-10 (IL-10)-producing splenocytes are generated early during infection and that IL-10 inhibits IFN-gamma-producing cells and delays the clearance of B. bronchiseptica from the lungs. The TTSS of B. bronchiseptica inhibits the generation of IFN-gamma-producing splenocytes and is required for long-term bacterial persistence in the lower respiratory tract in wild-type mice. This suggests that a mechanism involving the modulation of IFN-gamma production by the TTSS facilitates B. bronchiseptica survival in the lower respiratory tract.
Figures
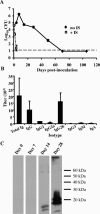
FIG. 1.
B. bronchiseptica can persist in the lower respiratory tract despite significant antibody titers. (A) Groups of three C57BL/6 mice were intranasally inoculated with 5 × 105 CFU of B. bronchiseptica and either given 200 μl of immune serum (IS) on day 0 (open squares) or left untreated (filled diamonds). Values are represented as the means ± standard errors. The dashed line indicates the limit of detection. (B) Titers of _B. bronchiseptica_-specific antibodies were measured by ELISA in serum samples collected on day 28 from _B. bronchiseptica_-infected C57BL/6 mice. Values are represented as the means ± standard errors. (C) Western blot of B. bronchiseptica lysate probed with lung homogenate collected from _B. bronchiseptica_-infected C57BL/6 mice on days 0, 7, 14, and 28 postinoculation. Data are shown for one of two replicates of these experiments, which both gave similar results.
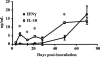
FIG. 2.
IL-10-producing splenocytes are generated early during infection. Splenocytes were collected from groups of three to four C57BL/6 mice at the indicated time points after intranasal inoculation with 5 × 105 CFU of B. bronchiseptica. The splenocytes were restimulated with HK B. bronchiseptica for 3 days and the supernatants were analyzed for IFN-γ (filled diamonds) and IL-10 (open squares) by ELISA. Data are shown for one of two replicates, which both gave similar results. Values are represented as the means ± standard errors. *, P < 0.05. Statistical significance was determined by comparing the levels of IL-10 and IFN-γ produced by splenocytes from _B. bronchiseptica_-infected mice to the levels of the respective cytokines produced by splenocytes from uninfected mice.
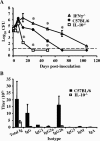
FIG. 3.
IFN-γ contributes to B. bronchiseptica clearance, while IL-10 inhibits clearance. Groups of three to four C57BL/6 mice, IFN-γ −/− mice, or IL-10−/− mice were intranasally inoculated with 5 × 105 CFU of B. bronchiseptica. (A) Bacterial numbers in the lungs were determined at the indicated time points postinoculation. Shown are C57BL/6 mice (filled diamonds), IFN-γ −/− mice (open triangles), and IL-10−/− mice (open squares). The dashed line indicates the limit of detection. *, P < 0.05. (B) _B. bronchiseptica_-specific antibody titers in serum collected from C57BL/6 (filled squares) or IL-10−/− (open squares) mice on day 28 postinoculation were measured by ELISA. Values are represented as the means ± standard errors. The experiment was replicated twice.
FIG. 4.
IL-10 inhibits the generation of IFN-γ-producing splenocytes. Splenocytes were collected from groups of three to four C57BL/6 (filled diamonds) or IL-10−/− (open squares) mice at the indicated time points after intranasal inoculation with 5 × 105 CFU of B. bronchiseptica. The splenocytes were restimulated with HK B. bronchiseptica for 3 days and the supernatants were analyzed for IFN-γ. The experiment was replicated twice. Values are represented as the means ± standard errors. *, P < 0.05.
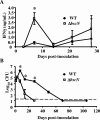
FIG. 5.
The TTSS inhibits IFN-γ production and is required for the persistence of B. bronchiseptica in the lower respiratory tract. (A) Groups of three to four C57BL/6 mice were intranasally inoculated with 5 × 105 CFU of the wild-type (filled diamonds) or Δ_bscN_ (open squares) strain of B. bronchiseptica. The splenocytes were collected at the indicated time points postinoculation and restimulated for 3 days with HK B. bronchiseptica, and the supernatant was analyzed for IFN-γ by ELISA. (B) The bacterial numbers in the lungs were determined at the indicated time points postinoculation. Values are represented as the means ± standard errors. The dashed line indicates the limit of detection. *, P < 0.05. WT, wild type.
Similar articles
- Bordetella type III secretion modulates dendritic cell migration resulting in immunosuppression and bacterial persistence.
Skinner JA, Pilione MR, Shen H, Harvill ET, Yuk MH. Skinner JA, et al. J Immunol. 2005 Oct 1;175(7):4647-52. doi: 10.4049/jimmunol.175.7.4647. J Immunol. 2005. PMID: 16177111 - Membrane Vesicles Derived from Bordetella bronchiseptica: Active Constituent of a New Vaccine against Infections Caused by This Pathogen.
Bottero D, Zurita ME, Gaillard ME, Bartel E, Vercellini C, Hozbor D. Bottero D, et al. Appl Environ Microbiol. 2018 Jan 31;84(4):e01877-17. doi: 10.1128/AEM.01877-17. Print 2018 Feb 15. Appl Environ Microbiol. 2018. PMID: 29180369 Free PMC article. - pagP is required for resistance to antibody-mediated complement lysis during Bordetella bronchiseptica respiratory infection.
Pilione MR, Pishko EJ, Preston A, Maskell DJ, Harvill ET. Pilione MR, et al. Infect Immun. 2004 May;72(5):2837-42. doi: 10.1128/IAI.72.5.2837-2842.2004. Infect Immun. 2004. PMID: 15102794 Free PMC article. - Environmental sensing mechanisms in Bordetella.
Coote JG. Coote JG. Adv Microb Physiol. 2001;44:141-81. doi: 10.1016/s0065-2911(01)44013-6. Adv Microb Physiol. 2001. PMID: 11407112 Review. - Eosinophils as drivers of bacterial immunomodulation and persistence.
Parrish KM, Gestal MC. Parrish KM, et al. Infect Immun. 2024 Sep 10;92(9):e0017524. doi: 10.1128/iai.00175-24. Epub 2024 Jul 15. Infect Immun. 2024. PMID: 39007622 Free PMC article. Review.
Cited by
- O antigen allows B. parapertussis to evade B. pertussis vaccine-induced immunity by blocking binding and functions of cross-reactive antibodies.
Zhang X, Rodríguez ME, Harvill ET. Zhang X, et al. PLoS One. 2009 Sep 14;4(9):e6989. doi: 10.1371/journal.pone.0006989. PLoS One. 2009. PMID: 19750010 Free PMC article. - Lack of cross-protection against Bordetella holmesii after pertussis vaccination.
Zhang X, Weyrich LS, Lavine JS, Karanikas AT, Harvill ET. Zhang X, et al. Emerg Infect Dis. 2012 Nov;18(11):1771-9. doi: 10.3201/eid1811.111544. Emerg Infect Dis. 2012. PMID: 23092514 Free PMC article. - Conquering the host: Bordetella spp. and Pseudomonas aeruginosa molecular regulators in lung infection.
Holban AM, Gregoire CM, Gestal MC. Holban AM, et al. Front Microbiol. 2022 Sep 26;13:983149. doi: 10.3389/fmicb.2022.983149. eCollection 2022. Front Microbiol. 2022. PMID: 36225372 Free PMC article. Review. - Constraint-based network model of pathogen-immune system interactions.
Thakar J, Saadatpour-Moghaddam A, Harvill ET, Albert R. Thakar J, et al. J R Soc Interface. 2009 Jul 6;6(36):599-612. doi: 10.1098/rsif.2008.0363. Epub 2008 Oct 24. J R Soc Interface. 2009. PMID: 18952547 Free PMC article. - A Type VI secretion system encoding locus is required for Bordetella bronchiseptica immunomodulation and persistence in vivo.
Weyrich LS, Rolin OY, Muse SJ, Park J, Spidale N, Kennett MJ, Hester SE, Chen C, Dudley EG, Harvill ET. Weyrich LS, et al. PLoS One. 2012;7(10):e45892. doi: 10.1371/journal.pone.0045892. Epub 2012 Oct 12. PLoS One. 2012. PMID: 23071529 Free PMC article.
References
- Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502-507. - PubMed
- Brady, M. T., A. J. MacDonald, A. G. Rowan, and K. H. Mills. 2003. Hepatitis C virus non-structural protein 4 suppresses Th1 responses by stimulating IL-10 production from monocytes. Eur. J. Immunol. 33:3448-3457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases