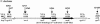Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system - PubMed (original) (raw)
Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system
Stefan Pukatzki et al. Proc Natl Acad Sci U S A. 2006.
Abstract
The bacterium Vibrio cholerae, like other human pathogens that reside in environmental reservoirs, survives predation by unicellular eukaryotes. Strains of the O1 and O139 serogroups cause cholera, whereas non-O1/non-O139 strains cause human infections through poorly defined mechanisms. Using Dictyostelium discoideum as a model host, we have identified a virulence mechanism in a non-O1/non-O139 V. cholerae strain that involves extracellular translocation of proteins that lack N-terminal hydrophobic leader sequences. Accordingly, we have named these genes "VAS" genes for virulence-associated secretion, and we propose that these genes encode a prototypic "type VI" secretion system. We show that vas genes are required for cytotoxicity of V. cholerae cells toward Dictyostelium amoebae and mammalian J774 macrophages by a contact-dependent mechanism. A large number of Gram-negative bacterial pathogens carry genes homologous to vas genes and potential effector proteins secreted by this pathway (i.e., hemolysin-coregulated protein and VgrG). Mutations in vas homologs in other bacterial species have been reported to attenuate virulence in animals and cultured macrophages. Thus, the genes encoding the VAS-related, type VI secretion system likely play an important conserved function in microbial pathogenesis and represent an additional class of targets for vaccine and antimicrobial drug-based therapies.
Figures
Fig. 1.
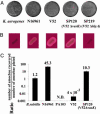
V. cholerae cytotoxicity toward the simple eukaryote D. discoideum. (A) Plaque assay. D. discoideum cells were plated on SM/5 with K. aerogenes and V. cholerae strains N16961, V52, SP120 (V52Δ_vasK_), and SP219 (V52Δ_hlyA_) at a density of ≈100 amoebae per plate. Bacterial virulence potential was determined by the number of plaques formed by D. discoideum in bacterial lawns. (B) Hemolytic phenotype of K. aerogenes and V. cholerae strains N16961, V52, SP120, and SP219 on trypticase soy agar containing 5% sheep blood. (C) Killing assay. Virulence of indicated bacteria was determined by enumerating the number of live amoebae recovered from bacterial lawns after a 24-h incubation. Numbers above the columns indicate fold change of number of amoebae in bacterial lawns over a 24-h period. Results shown are the means (±SD) of triplicate determinations.
Fig. 2.
Genetic organization of the VAS pathway of V. cholerae. Horizontal gray arrows designate hypothetical genes, black arrows designate genes with homologues of known function, and empty arrows indicate genes of known function in V. cholerae (drawn to scale). Vertical arrows indicate transposon insertion sites in _Dictyostelium_-attenuated V. cholerae mutants.
Fig. 3.
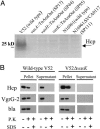
VAS-dependent secretion. (A) Secretion profiles of V. cholerae VAS mutants. SDS/PAGE of concentrated midlog culture supernatants of indicated strains. Black arrow indicates position of Hcp. (B) Extracellular secretion of epitope-tagged substrates. V. cholerae strains V52 and SP120 (V52Δ_vasK_) maintaining a plasmid that allows arabinose-induced expression of tagged Hcp-2 and VgrG-2 were grown under inducing conditions. Cells and filtered supernatants were left untreated or incubated with either 0.1 mg/ml proteinase K (P.K) in the presence or absence of 1% SDS. Protease inhibitor PMSF was used to stop proteolysis after 20 min, and extracts were separated on a SDS/PAGE for immunoblotting with vesicular stomatitis virus glycoprotein antisera. The quality of pellet and supernatant fractionation was determined by localizing periplasmic β-lactamase (bla).
Fig. 4.
V. cholerae cytotoxicity toward J774 macrophages. J774 cells were infected for 2 h with V52 (wild type) and isogenic mutant SP500 (Δ_rtxA_, Δ_hlyA_) or mutant SP501 (Δ_rtxA_, Δ_hlyA_, Δ_vasK_). Cells were fixed with 3% paraformaldehyde to assess the morphology of infected cells.
Similar articles
- Genetic analysis of anti-amoebae and anti-bacterial activities of the type VI secretion system in Vibrio cholerae.
Zheng J, Ho B, Mekalanos JJ. Zheng J, et al. PLoS One. 2011;6(8):e23876. doi: 10.1371/journal.pone.0023876. Epub 2011 Aug 31. PLoS One. 2011. PMID: 21909372 Free PMC article. - [Study of expression of contact-dependent secretion systems in Vibrio cholerae on the model of Dictyostelium discoideum].
Monakhova EV, Bozhko NV. Monakhova EV, et al. Zh Mikrobiol Epidemiol Immunobiol. 2010 Jul-Aug;(4):89-92. Zh Mikrobiol Epidemiol Immunobiol. 2010. PMID: 20799403 Russian. - Clinical and environmental isolates of Vibrio cholerae serogroup O141 carry the CTX phage and the genes encoding the toxin-coregulated pili.
Dalsgaard A, Serichantalergs O, Forslund A, Lin W, Mekalanos J, Mintz E, Shimada T, Wells JG. Dalsgaard A, et al. J Clin Microbiol. 2001 Nov;39(11):4086-92. doi: 10.1128/JCM.39.11.4086-4092.2001. J Clin Microbiol. 2001. PMID: 11682534 Free PMC article. - Epidemiology & molecular biology of Vibrio cholerae O139 Bengal.
Albert MJ. Albert MJ. Indian J Med Res. 1996 Jul;104:14-27. Indian J Med Res. 1996. PMID: 8783504 Review. - Expression of Vibrio cholerae virulence genes in response to environmental signals.
Peterson KM. Peterson KM. Curr Issues Intest Microbiol. 2002 Sep;3(2):29-38. Curr Issues Intest Microbiol. 2002. PMID: 12400636 Review.
Cited by
- Quorum sensing orchestrates parallel cell death pathways in Vibrio cholerae via Type 6 secretion-dependent and -independent mechanisms.
Mashruwala AA, Bassler BL. Mashruwala AA, et al. Proc Natl Acad Sci U S A. 2024 Nov 12;121(46):e2412642121. doi: 10.1073/pnas.2412642121. Epub 2024 Nov 5. Proc Natl Acad Sci U S A. 2024. PMID: 39499633 Free PMC article. - Gamma-Mobile-Trio systems are mobile elements rich in bacterial defensive and offensive tools.
Mahata T, Kanarek K, Goren MG, Marimuthu Ragavan R, Bosis E, Qimron U, Salomon D. Mahata T, et al. Nat Microbiol. 2024 Dec;9(12):3268-3283. doi: 10.1038/s41564-024-01840-5. Epub 2024 Oct 23. Nat Microbiol. 2024. PMID: 39443754 - Vibrio cholerae: a fundamental model system for bacterial genetics and pathogenesis research.
van Kessel JC, Camilli A. van Kessel JC, et al. J Bacteriol. 2024 Nov 21;206(11):e0024824. doi: 10.1128/jb.00248-24. Epub 2024 Oct 15. J Bacteriol. 2024. PMID: 39405459 Free PMC article. Review. - Quorum sensing orchestrates parallel cell death pathways in Vibrio cholerae via Type 6 secretion dependent and independent mechanisms.
Mashruwala AA, Bassler BL. Mashruwala AA, et al. bioRxiv [Preprint]. 2024 Sep 23:2024.09.23.614608. doi: 10.1101/2024.09.23.614608. bioRxiv. 2024. PMID: 39386452 Free PMC article. Updated. Preprint. - Quorum regulated latent environmental cells of toxigenic Vibrio cholerae and their role in cholera outbreaks.
Faruque SN, Yamasaki S, Faruque SM. Faruque SN, et al. Gut Pathog. 2024 Sep 29;16(1):52. doi: 10.1186/s13099-024-00647-3. Gut Pathog. 2024. PMID: 39343919 Free PMC article. Review.
References
- Zinnaker, Y. & Carpenter, C. C. (1972) Johns Hopkins Med. 131, 403-411. - PubMed
- Steinert, M. & Heuner, K. (2005) Cell. Microbiol. 7, 307-314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI 18045/AI/NIAID NIH HHS/United States
- R37 AI018045/AI/NIAID NIH HHS/United States
- R01 AI018045/AI/NIAID NIH HHS/United States
- N01 AI 30071/AI/NIAID NIH HHS/United States
- N01AI30071/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases