Protocol of the COSMIN study: COnsensus-based Standards for the selection of health Measurement INstruments - PubMed (original) (raw)
Protocol of the COSMIN study: COnsensus-based Standards for the selection of health Measurement INstruments
L B Mokkink et al. BMC Med Res Methodol. 2006.
Abstract
Background: Choosing an adequate measurement instrument depends on the proposed use of the instrument, the concept to be measured, the measurement properties (e.g. internal consistency, reproducibility, content and construct validity, responsiveness, and interpretability), the requirements, the burden for subjects, and costs of the available instruments. As far as measurement properties are concerned, there are no sufficiently specific standards for the evaluation of measurement properties of instruments to measure health status, and also no explicit criteria for what constitutes good measurement properties. In this paper we describe the protocol for the COSMIN study, the objective of which is to develop a checklist that contains COnsensus-based Standards for the selection of health Measurement INstruments, including explicit criteria for satisfying these standards. We will focus on evaluative health related patient-reported outcomes (HR-PROs), i.e. patient-reported health measurement instruments used in a longitudinal design as an outcome measure, excluding health care related PROs, such as satisfaction with care or adherence. The COSMIN standards will be made available in the form of an easily applicable checklist.
Method: An international Delphi study will be performed to reach consensus on which and how measurement properties should be assessed, and on criteria for good measurement properties. Two sources of input will be used for the Delphi study: (1) a systematic review of properties, standards and criteria of measurement properties found in systematic reviews of measurement instruments, and (2) an additional literature search of methodological articles presenting a comprehensive checklist of standards and criteria. The Delphi study will consist of four (written) Delphi rounds, with approximately 30 expert panel members with different backgrounds in clinical medicine, biostatistics, psychology, and epidemiology. The final checklist will subsequently be field-tested by assessing the inter-rater reproducibility of the checklist.
Discussion: Since the study will mainly be anonymous, problems that are commonly encountered in face-to-face group meetings, such as the dominance of certain persons in the communication process, will be avoided. By performing a Delphi study and involving many experts, the likelihood that the checklist will have sufficient credibility to be accepted and implemented will increase.
Figures
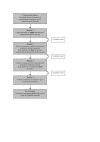
Figure 1
the Delphi procedure of the COSMIN study.
Similar articles
- Quality criteria were proposed for measurement properties of health status questionnaires.
Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, Bouter LM, de Vet HC. Terwee CB, et al. J Clin Epidemiol. 2007 Jan;60(1):34-42. doi: 10.1016/j.jclinepi.2006.03.012. Epub 2006 Aug 24. J Clin Epidemiol. 2007. PMID: 17161752 - Procedures and methods of benefit assessments for medicines in Germany.
Bekkering GE, Kleijnen J. Bekkering GE, et al. Eur J Health Econ. 2008 Nov;9 Suppl 1:5-29. doi: 10.1007/s10198-008-0122-5. Eur J Health Econ. 2008. PMID: 18987905 - [Procedures and methods of benefit assessments for medicines in Germany].
Bekkering GE, Kleijnen J. Bekkering GE, et al. Dtsch Med Wochenschr. 2008 Dec;133 Suppl 7:S225-46. doi: 10.1055/s-0028-1100954. Epub 2008 Nov 25. Dtsch Med Wochenschr. 2008. PMID: 19034813 German. - Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria.
Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Evers S, et al. Int J Technol Assess Health Care. 2005 Spring;21(2):240-5. Int J Technol Assess Health Care. 2005. PMID: 15921065 Review. - Evaluating patient-reported outcome measures (PROMs) for bladder cancer: a systematic review using the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) checklist.
Mason SJ, Catto JWF, Downing A, Bottomley SE, Glaser AW, Wright P. Mason SJ, et al. BJU Int. 2018 Nov;122(5):760-773. doi: 10.1111/bju.14368. Epub 2018 Jun 8. BJU Int. 2018. PMID: 29726085 Free PMC article.
Cited by
- Orthodontic treatment during pregnancy, lactation, and postmenopausal period: a questionnaire development.
Fernandes JL, Perazzo MF, Paiva SM, Martins-Júnior PA, Macari S. Fernandes JL, et al. Braz Oral Res. 2024 Jan 5;38:e013. doi: 10.1590/1807-3107bor-2024.vol38.0013. eCollection 2024. Braz Oral Res. 2024. PMID: 38198311 Free PMC article. - Responsiveness, construct and criterion validity of the Personal Care-Participation Assessment and Resource Tool (PC-PART).
Darzins SW, Imms C, Shields N, Taylor NF. Darzins SW, et al. Health Qual Life Outcomes. 2015 Aug 12;13:125. doi: 10.1186/s12955-015-0322-5. Health Qual Life Outcomes. 2015. PMID: 26264043 Free PMC article. Clinical Trial. - Physical Activity Questionnaires for Pregnancy: A Systematic Review of Measurement Properties.
Sattler MC, Jaunig J, Watson ED, van Poppel MNM, Mokkink LB, Terwee CB, Dietz P. Sattler MC, et al. Sports Med. 2018 Oct;48(10):2317-2346. doi: 10.1007/s40279-018-0961-x. Sports Med. 2018. PMID: 30094797 Free PMC article. - Assessment of Quality-of-Life Measurement Instruments for Chronic Otitis Media: A Systematic Review Using the COnsensus-Based Standards for the Selection of Health Measurement Instruments (COSMIN) Checklist.
Devi NS, Kumar A, G V, Sood R, Tyagi AK, Jat B, Patro S, Jayaprakash PA, Prasath R, Yadav AC, Nivedhan R. Devi NS, et al. Indian J Otolaryngol Head Neck Surg. 2025 Feb;77(2):699-710. doi: 10.1007/s12070-024-05224-3. Epub 2024 Nov 22. Indian J Otolaryngol Head Neck Surg. 2025. PMID: 40070742 - Systematic review of validated parent-reported questionnaires assessing swallowing dysfunction in otherwise healthy infants and toddlers.
Baqays A, Zenke J, Campbell S, Johannsen W, Rashid M, Seikaly H, El-Hakim H. Baqays A, et al. J Otolaryngol Head Neck Surg. 2021 Dec 4;50(1):68. doi: 10.1186/s40463-021-00549-3. J Otolaryngol Head Neck Surg. 2021. PMID: 34863293 Free PMC article.
References
- Bombardier C, Tugwell P. Methodological considerations in functional assessment. J Rheumatol Suppl. 1987;14 Suppl 15:6–10. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous