The distribution of intermediate-conductance, calcium-activated, potassium (IK) channels in epithelial cells - PubMed (original) (raw)
The distribution of intermediate-conductance, calcium-activated, potassium (IK) channels in epithelial cells
Nichola Thompson-Vest et al. J Anat. 2006 Feb.
Abstract
Intermediate-conductance, calcium-activated, potassium (IK) channels were first identified by their roles in cell volume regulation, and were later shown to be involved in control of proliferation of lymphocytes and to provide a K+ current for epithelial secretory activity. Until now, there has been no systematic investigation of IK channel localization within different epithelia. IK channel immunoreactivity was present in most epithelia, where it occurred in surface membranes of epithelial cells. It was found in all stratified epithelia, including skin, cornea, oral mucosa, vaginal mucosa, urothelium and the oesophageal lining. It occurred in the ducts of fluid-secreting glands, the salivary glands, lacrimal glands and pancreas, and in the respiratory epithelium. A low level of expression was seen in serous acinar cells. It was also found in other epithelia with fluid-exchange properties, the choroid plexus epithelium, the ependyma, visceral pleura and peritoneum, bile ducts and intestinal lining epithelium. However, there was little or no expression in vascular endothelial cells, kidney tubules or collecting ducts, lung alveoli, or in sebaceous glands. It is concluded that the channel is present in surface epithelia (e.g. skin) where it has a cell-protective role against osmotic challenge, and in epithelia where there is anion secretion that is facilitated by a K+ current-dependent hyperpolarization. It was also in some epithelial cells where its roles are as yet unknown.
Figures
Fig. 1
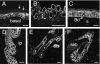
Examples of IK channel immunoreactivity in stratified squamous epithelia. (A) Thin skin from the ear. The cells of the basal and spiny cell layers have cell surface immunoreactivity. Cells of the stratum corneum (arrows) are not IK channel immunoreactive. (B) Oral mucosa, showing IK channel immunoreactivity of cells of the basal layers. (C) Cornea. Cells through the full thickness of the cornea have channel immunoreactivity. The underlying Bowman's membrane (Bo) and the corneal stroma (s) are unstained. (D) Vagina. Cells through the full thickness of the epithelium are immunoreactive. Cells in the lamina propria (lp) are not labelled. (E) Hair follicles and dermis. Immunoreactivity can be seen in the dermal invaginations that form the walls of the follicles, but does not occur in the hair root (r) or hair shaft (s). (F) Sebaceous gland (seb) and part of the hair follicle into which it drains. No immunoreactivity occurs in the sebaceous gland, but immunoreactive epithelial cells of the follicle are observed. Scale bars: 20 µm.
Fig. 2
IK channel immunoreactivity in the respiratory epithelium. (A) The immunoreactivity occurs in the lining of the trachea. It is prominent in the pyramidal basal cells (arrows). The lamina propria (lp) and smooth muscle (sm) are not reactive. (B) Higher magnification view of the tracheal epithelium. IK channel immunoreactivity is seen at the surface membranes of basal cells (arrow) and in the basolateral membranes of the columnar cells (arrowhead). Goblet cells were not immunoreactive (cell adjacent to asterisk). One of the columnar cells is shown at higher magnification (lower micrograph). Note the absence of labelling of the apical membrane (arrow). (C) Immunoreactivity of the cuboidal cells of the bronchial epithelium. The underlying cartilage (cart) is not stained. (D) Bronchial epithelium at higher magnification. Immunoreactivity is observed on the basolateral membranes, and weakly in the cytoplasm (cell adjacent to asterisk). (E) Immunoreactivity of the simple squamous epithelium of the visceral pleural membrane. Note that the alveoli (alv) adjacent to the pleural membrane are not immunoreactive. Scale bars: 20 µm.
Fig. 3
Blood vessels, intra-hepatic biliary ducts and visceral peritoneum. (A) Branches of the hepatic portal vein (vein), hepatic artery (art) and bile duct (bil) in the parenchyma of the liver. The only IK channel immunoreactivity is in the epithelium of the intra-hepatic biliary duct, which is shown at higher magnification in the inset. The endothelial cells of the vein and artery are negative, as are the hepatocytes. (B) The central vein and bile ductules. The epithelium of the ductules (d) is positively stained, but the endothelium of the central vein is unreactive. (C) Cross-section through a small mesenteric artery. Very faint staining is present in endothelial cells of the intima (arrows). (D) IK channel immunoreactivity of the simple squamous epithelium (visceral peritoneum) on the surface of the spleen. Peritoneal space: perit space. The parenchyma of the spleen itself (par) was negative. Scale bars: 20 µm.
Fig. 4
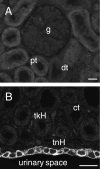
IK channel immunoreactivity in the kidney. (A) Renal cortex showing the lack of immunoreactivity of a glomerulus (g), the proximal convoluted tubules (pt) and distal convoluted tubules (dt). (B) The renal medulla, with lack of IK staining in thick loops of Henle (tkH), thin loops (tnH) and collecting tubules (ct). The urothelium at the inner surface of the renal medulla, adjacent to the urinary space (urinary space), is immunoreactive. Scale bar: 20 µm.
Fig. 5
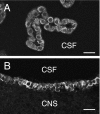
Epithelial cells of the choroid plexus and ependyma, showing IK channel immunoreactivity. (A) A region of choroid plexus from the fourth ventricle. The cuboidal cells facing the cerebrospinal fluid (CSF) space are immunoreactive, but no reaction is observed in the underlying vascular endothelium. (B) Immunoreactivity of the ependymal cells lining the third ventricle. Note the lack of immunoreactivity of the adjacent central nervous system (CNS). Scale bars: 20 µm.
Fig. 6
IK channel immunoreactivity in the walls of fluid-secreting glands and ducts. (A) Section through the pancreas. Immunoreactivity is seen in the intercalated ducts (arrows) and in an intra-pancreatic duct (duct). (B) Interlobular duct of the extra-orbital lacrimal gland, showing IK channel immunoreactivity of the duct epithelium. (C) Intercalated ducts shown in longitudinal and in cross-section in the submandibular gland. In the cross-section of the duct, immunoreactivity is clearly seen at the apical surface. (D) Continuity between an intercalated duct (arrow) and a striated duct (arrowheads) in the submandibular gland. The cells forming the walls of the striated duct were immunoreactive. (E) Acinar cells of the parotid gland, showing IK channel immunoreactivity at the basolateral surfaces. (F) The wall of the extra-hepatic bile duct, showing its immunoreactive epithelium. (G) Eccrine sweat gland from the rat paw pad. A low level of IK channel immunoreactivity is seen. Scale bars: 20 µm.
Fig. 7
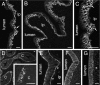
Epithelia of urinogenital organs. The lumen and lamina propria (lp) are indicated. (A) Transitional epithelium of the ureter. Immunoreactivity occurs at the surface membranes of the basal epithelial cells (arrow). Slight protein autofluorescence reveals the luminal surface of the epithelium. (B) Epithelium lining the bladder. Note that there is no immunoreactivity of the underlying connective tissue of the lamina propria (lp) or muscle. (C) IK channel immunoreactivity in the urethra. Cells of the basal layer and adjacent layers are immunoreactive. (D) Immunoreactivity in the epidydimis. The only prominent immunoreactivity is in the basal cells of the epithelium. Part of an epidydimal duct is shown at higher magnification in the inset. The tall columnar cells (c) of the epidydimal lining were unstained. (E) Vas deferens. The epithelial cells show immunoreactivity of their surface membranes. Basal cells (arrowheads) are more strongly immunoreactive than columnar cells. The underlying lamina propria (lp) and smooth muscle were not immunoreactive. (F) Fallopian tube. The IK channel immunoreactivity is seen in the basolateral membranes, but the cilia are not reactive. (G) Uterus. The columnar epithelial cells exhibit IK channel immunoreactivity, but the underlying stroma (s) is not immunoreactive. Scale bars: 20 µm.
Fig. 8
Evidence for IK channel protein and gene expression in epithelia. Samples were taken from the urothelium and, for comparison, from the external muscle/myenteric plexus of the intestine, where IK channels occur in neurons. (A) Western blot analysis of protein extracted from the epithelium of the urinary bladder shows a single band at approximately 50 kDa, the predicted size of the channel protein monomer. (B) RT-PCR product from an extract of urinary bladder endothelium and from the colon, showing bands of the predicted size.
Similar articles
- Expression of intermediate conductance potassium channel immunoreactivity in neurons and epithelial cells of the rat gastrointestinal tract.
Furness JB, Robbins HL, Selmer IS, Hunne B, Chen MX, Hicks GA, Moore S, Neylon CB. Furness JB, et al. Cell Tissue Res. 2003 Nov;314(2):179-89. doi: 10.1007/s00441-003-0808-z. Epub 2003 Sep 26. Cell Tissue Res. 2003. PMID: 14513356 - Colocalization of 11 beta-hydroxysteroid dehydrogenase type II and mineralocorticoid receptor in human epithelia.
Hirasawa G, Sasano H, Takahashi K, Fukushima K, Suzuki T, Hiwatashi N, Toyota T, Krozowski ZS, Nagura H. Hirasawa G, et al. J Clin Endocrinol Metab. 1997 Nov;82(11):3859-63. doi: 10.1210/jcem.82.11.4337. J Clin Endocrinol Metab. 1997. PMID: 9360552 - Mechanisms of cellular synchronization in the vascular wall. Mechanisms of vasomotion.
Matchkov VV. Matchkov VV. Dan Med Bull. 2010 Oct;57(10):B4191. Dan Med Bull. 2010. PMID: 21040688 Review. - Ca²⁺-dependent K⁺ channels in exocrine salivary glands.
Catalán MA, Peña-Munzenmayer G, Melvin JE. Catalán MA, et al. Cell Calcium. 2014 Jun;55(6):362-8. doi: 10.1016/j.ceca.2014.01.005. Epub 2014 Jan 31. Cell Calcium. 2014. PMID: 24559652 Free PMC article. Review.
Cited by
- Molecular basis of potassium channels in pancreatic duct epithelial cells.
Hayashi M, Novak I. Hayashi M, et al. Channels (Austin). 2013 Nov-Dec;7(6):432-41. doi: 10.4161/chan.26100. Epub 2013 Aug 20. Channels (Austin). 2013. PMID: 23962792 Free PMC article. Review. - Functional coupling of TRPV4, IK, and SK channels contributes to Ca(2+)-dependent endothelial injury in rodent lung.
Lin MT, Jian MY, Taylor MS, Cioffi DL, Yap FC, Liedtke W, Townsley MI. Lin MT, et al. Pulm Circ. 2015 Jun;5(2):279-90. doi: 10.1086/680166. Pulm Circ. 2015. PMID: 26064452 Free PMC article. - Identification and functional characterization of the intermediate-conductance Ca(2+)-activated K(+) channel (IK-1) in biliary epithelium.
Dutta AK, Khimji AK, Sathe M, Kresge C, Parameswara V, Esser V, Rockey DC, Feranchak AP. Dutta AK, et al. Am J Physiol Gastrointest Liver Physiol. 2009 Nov;297(5):G1009-18. doi: 10.1152/ajpgi.00223.2009. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 20501432 Free PMC article. - Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases.
Dartt DA. Dartt DA. Prog Retin Eye Res. 2009 May;28(3):155-77. doi: 10.1016/j.preteyeres.2009.04.003. Epub 2009 Apr 17. Prog Retin Eye Res. 2009. PMID: 19376264 Free PMC article. Review. - Advances in Ca2+ modulation of gastrointestinal anion secretion and its dysregulation in digestive disorders (Review).
Shan W, Hu Y, Ding J, Yang X, Lou J, Du Q, Liao Q, Luo L, Xu J, Xie R. Shan W, et al. Exp Ther Med. 2020 Nov;20(5):8. doi: 10.3892/etm.2020.9136. Epub 2020 Aug 25. Exp Ther Med. 2020. PMID: 32934673 Free PMC article. Review.
References
- Begenisich T, Nakamoto T, Ovitt C, et al. Physiological roles of the intermediate conductance, Ca2+-activated K channel, Kcnn4. J Biol Chem. 2004;279:47681–47687. - PubMed
- Chen MX, Gorman SA, Benson B, et al. Small and intermediate conductance Ca2+-activated K+ channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum. Naunyn-Schmiedeberg's Arch Pharmacol. 2004;369:602–615. - PubMed
- Cuthbert AW, Hickman ME, Thorn P, MacVinish LJ. Activation of Ca2+- and cAMP-sensitive K+ channels in murine colonic epithelia by 1-ethyl-2-benzimidazolone. Am J Physiol. 1999;277:C111–C120. - PubMed
- Furness JB, Robbins HL, Selmer I-S, et al. Expression of intermediate conductance potassium channel immunoreactivity in neurons and epithelial cells of the rat gastrointestinal tract. Cell Tissue Res. 2003;314:179–189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources