Lesions of the habenula produce stress- and dopamine-dependent alterations in prepulse inhibition and locomotion - PubMed (original) (raw)
Comparative Study
Lesions of the habenula produce stress- and dopamine-dependent alterations in prepulse inhibition and locomotion
Scott A Heldt et al. Brain Res. 2006.
Abstract
The habenula complex modulates the activity of dopamine and serotonin systems in the brain. An important question remains whether there is a link between habenula dysfunction and monoamine-related disorders, such as schizophrenia. In this study, we describe an interaction between habenula lesions and stress that produces long-lasting effects on behavior. Mice received control lesions or bilateral electrolytic lesions of the habenula and were tested for fear-potentiated startle and freezing measures of conditioned fear. They were also tested for prepulse inhibition (PPI) and locomotor activity in the presence or absence of a dopaminergic agonist (apomorphine) or an atypical antipsychotic with mixed dopamine/serotonin antagonist properties (clozapine). There were no detectable effects of habenula lesions on fear conditioning and no effects on PPI in the absence of stress. However, following conditioned fear stress, habenula-lesioned animals showed decreased PPI which normalized with clozapine. Lesioned animals also showed diminished activity at baseline, with hyperlocomotion following apomorphine. These data support the hypothesis that the habenula may be normally involved in stress-dependent regulation of monoamine systems.
Figures
Fig. 1
(a) Representative photomicrographs of sham-lesioned animals (top row) and habenula-lesioned animals (bottom row). Arrows demonstrate location of habenula in control and lesioned animals. (b) Serial histological reconstruction of electrolytic lesions of the habenula in mice included in study. Reconstructions have been transcribed onto modified plates 40, 41, 43, 45, 47, and 49 as adapted from Paxinos and Franklin (2001) and illustrate the largest (light grey) and smallest (dark grey) lesions included in statistical analyses.
Fig. 2
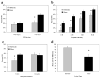
(a) Pre- and postsurgery PPI sessions designed to examine the effects of habenula damage on PPI. Mean percent PPI scores represent the average inhibition computed over 4 prepulse intensities (2, 4, 8, or 10 dB above a 63 dB white noise background). Postsurgery test was performed 1 week after surgery. (b) Freezing response to the presence of the tone conditioned stimulus (CS) during acquisition of conditioned fear. Training consisted of 5 tone + shock trials on each of 3 consecutive days (15 total trials). (c) Mean reactivity score in response to the 0.4 mA footshock used as the unconditioned stimulus (US) during fear conditioning. (d–e) Retention of conditioned fear as measured by (d) fear-potentiated startle and (e) freezing. The retention test was conducted 24 h after last training session. Bars represent means + SEM.
Fig. 3
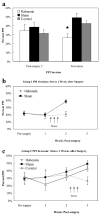
(a) Pre- and poststress PPI sessions designed to examine the effects of habenula damage and conditioned fear stress on PPI. Mean percent PPI scores represent the average inhibition computed over 4 prepulse intensities (2, 4, 8, or 10 dB). Poststress session was conducted 1 week after prestress session (2 week after surgery) during which time animals were given fear conditioning. (b) Mean percent PPI scores at each of the prepulse intensities. (c) Effects of clozapine on PPI in habenula- and sham-lesioned mice. Bars depict the mean percent PPI computed over 4 prepulse intensities. Mice were given i.p. injections of either vehicle or clozapine (6 mg/kg) 30 min before behavioral testing using a random crossover experimental design. (d) The effects of apomorphine on motor activity in habenula- and sham-lesioned animals. Bars represent mean increases in motor activity in response to apomorphine. The mean was computed by subtracting the number of compartment entries on session 1 (no drug, shams: M = 34.5, MSE = 3.7; habenula: M = 17.5, MSE = 2.3) from number of compartment entries on session 2 (apomorphine, shams: M = 87.3, MSE = 6.7; habenula: M = 104.5, MSE = 7.5). Apomorphine (2 mg/kg) was administered 30 min before session 2. Bars represent means + SEM (*_P_s < 0.05).
Fig. 4
(a) Mean percent PPI scores pre- and poststress for habenula, geniculate (control), and sham-lesioned animals. Poststress session was conducted 1 week after prestress session (2 week after surgery) during which time animals were given fear conditioning. Mean percent PPI scores represent the average inhibition averaged computed over 4 prepulse intensities (2, 4, 8, or 10 dB). (b–c) Comparison of mean percent PPI scores for Groups 1 and 2. Animals in Group 1 received stress between weeks 1 and 2 after lesion surgery. Group 2 animals that received stress between weeks 2 and 3 postsurgery (*P < 0.05).
Similar articles
- Inactivation of the 5-HT(7) receptor partially blocks phencyclidine-induced disruption of prepulse inhibition.
Semenova S, Geyer MA, Sutcliffe JG, Markou A, Hedlund PB. Semenova S, et al. Biol Psychiatry. 2008 Jan 1;63(1):98-105. doi: 10.1016/j.biopsych.2006.12.011. Epub 2007 May 24. Biol Psychiatry. 2008. PMID: 17531208 Free PMC article. - Reduced brain serotonin activity disrupts prepulse inhibition of the acoustic startle reflex. Effects of 5,7-dihydroxytryptamine and p-chlorophenylalanine.
Fletcher PJ, Selhi ZF, Azampanah A, Sills TL. Fletcher PJ, et al. Neuropsychopharmacology. 2001 Apr;24(4):399-409. doi: 10.1016/S0893-133X(00)00215-3. Neuropsychopharmacology. 2001. PMID: 11182535 - Effects of spiperone, raclopride, SCH 23390 and clozapine on apomorphine inhibition of sensorimotor gating of the startle response in the rat.
Swerdlow NR, Keith VA, Braff DL, Geyer MA. Swerdlow NR, et al. J Pharmacol Exp Ther. 1991 Feb;256(2):530-6. J Pharmacol Exp Ther. 1991. PMID: 1825226 - Angiotensin-converting enzyme (ACE) interacts with dopaminergic mechanisms in the brain to modulate prepulse inhibition in mice.
van den Buuse M, Zheng TW, Walker LL, Denton DA. van den Buuse M, et al. Neurosci Lett. 2005 May 20-27;380(1-2):6-11. doi: 10.1016/j.neulet.2005.01.009. Epub 2005 Jan 24. Neurosci Lett. 2005. PMID: 15854741 - Pharmacological analysis of fear-potentiated startle.
Davis M. Davis M. Braz J Med Biol Res. 1993 Mar;26(3):235-60. Braz J Med Biol Res. 1993. PMID: 8257926 Review.
Cited by
- Placing prediction into the fear circuit.
McNally GP, Johansen JP, Blair HT. McNally GP, et al. Trends Neurosci. 2011 Jun;34(6):283-92. doi: 10.1016/j.tins.2011.03.005. Epub 2011 May 5. Trends Neurosci. 2011. PMID: 21549434 Free PMC article. Review. - The Lateral Habenula Circuitry: Reward Processing and Cognitive Control.
Baker PM, Jhou T, Li B, Matsumoto M, Mizumori SJ, Stephenson-Jones M, Vicentic A. Baker PM, et al. J Neurosci. 2016 Nov 9;36(45):11482-11488. doi: 10.1523/JNEUROSCI.2350-16.2016. J Neurosci. 2016. PMID: 27911751 Free PMC article. Review. - Enduring sensorimotor gating abnormalities following predator exposure or corticotropin-releasing factor in rats: a model for PTSD-like information-processing deficits?
Bakshi VP, Alsene KM, Roseboom PH, Connors EE. Bakshi VP, et al. Neuropharmacology. 2012 Feb;62(2):737-48. doi: 10.1016/j.neuropharm.2011.01.040. Epub 2011 Feb 1. Neuropharmacology. 2012. PMID: 21288473 Free PMC article. - Role for the Rostromedial Tegmental Nucleus in Signaling the Aversive Properties of Alcohol.
Glover EJ, McDougle MJ, Siegel GS, Jhou TC, Chandler LJ. Glover EJ, et al. Alcohol Clin Exp Res. 2016 Aug;40(8):1651-61. doi: 10.1111/acer.13140. Epub 2016 Jul 8. Alcohol Clin Exp Res. 2016. PMID: 27388762 Free PMC article. - Genetic dissection of medial habenula-interpeduncular nucleus pathway function in mice.
Kobayashi Y, Sano Y, Vannoni E, Goto H, Suzuki H, Oba A, Kawasaki H, Kanba S, Lipp HP, Murphy NP, Wolfer DP, Itohara S. Kobayashi Y, et al. Front Behav Neurosci. 2013 Mar 12;7:17. doi: 10.3389/fnbeh.2013.00017. eCollection 2013. Front Behav Neurosci. 2013. PMID: 23487260 Free PMC article.
References
- Adell A, Garcia-Marquez C, Armario A, Gelpi E. Chronic stress increases serotonin and noradrenaline in rat brain and sensitizes their responses to a further acute stress. J Neurochem. 1988;50:1678–1681. - PubMed
- Amat J, Sparks PD, Matus-Amat P, Griggs J, Watkins LR, Maier SF. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 2001;917:118–126. - PubMed
- Andres KH, von During M, Veh RW. Subnuclear organization of the rat habenular complexes. J Comp Neurol. 1999;407:130–150. - PubMed
- Benabid AL, Jeaugey L. Cells of the rat lateral habenula respond to high-threshold somatosensory inputs. Neurosci Lett. 1989;96:289–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH069884/MH/NIMH NIH HHS/United States
- P51 RR000165/RR/NCRR NIH HHS/United States
- K01 MH069884-01/MH/NIMH NIH HHS/United States
- K01 MH069884/MH/NIMH NIH HHS/United States
- F32 MH073389/MH/NIMH NIH HHS/United States
- MH073389/MH/NIMH NIH HHS/United States
- P51 RR000165-440215/RR/NCRR NIH HHS/United States
- RR00165/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources