In situ labeling of immune cells with iron oxide particles: an approach to detect organ rejection by cellular MRI - PubMed (original) (raw)
In situ labeling of immune cells with iron oxide particles: an approach to detect organ rejection by cellular MRI
Yijen L Wu et al. Proc Natl Acad Sci U S A. 2006.
Abstract
In vivo cell tracking by MRI can provide means to observe biological processes and monitor cell therapy directly. Immune cells, e.g., macrophages, play crucial roles in many pathophysiological processes, including organ rejection, inflammation, autoimmune diseases, cancer, atherosclerotic plaque formation, numerous neurological disorders, etc. The current gold standard for diagnosing and staging rejection after organ transplantation is biopsy, which is not only invasive, but also prone to sampling errors. Here, we report a noninvasive approach using MRI to detect graft rejection after solid organ transplantation. In addition, we present the feasibility of imaging individual macrophages in vivo by MRI in a rodent heterotopic working-heart transplantation model using a more sensitive contrast agent, the micrometer-sized paramagnetic iron oxide particle, as a methodology to detect acute cardiac rejection.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
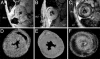
Fig. 1.
In vivo MRI of allograft hearts and lungs, 1 day after i.v. injection of MPIO particles. (A) Allograft heart on POD 5. (B and C) Allograft heart on POD 6. (D) Allograft lung on POD 6. (E) Isograft heart on POD 6. Shown with 156-μm in-plane resolution at 4.7 Tesla by using a Bruker Biospec AVANCE-DBX MRI instrument.
Fig. 2.
In vivo MRI of a rat allograft heart over time. MPIO particles are administered once on POD 3.5, and the same animal is imaged on POD 3.5 (A), 4.5 (B), and 5.5 (C), with 156-μm in-plane resolution at 4.7 Tesla by using a Bruker Biospec AVANCE-DBX MRI instrument.
Fig. 3.
Contrast patterns labeled with two different contrast agents. (A_–_C) In vivo MRI of macrophage accumulation on POD 6 of an allograft heart with MPIO-particle labeling (A), an isograft heart with MPIO-particle labeling (B), and an allograft heart with USPIO labeling (C), with 156-μm in-plane resolution at 4.7 Tesla. (D_–_F) MRM at 11.7 Tesla using a Bruker AVANCE-DBX MRI instrument with in-plane resolution of 40 μm of an allograft heart with MPIO-particle labeling (D), an isograft heart (E), and an allograft heart with USPIO labeling (F). All hearts used in the in vivo and ex vivo measurements were the same ones except those used in A and D.
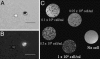
Fig. 4.
Ex-vivo labeled macrophages. Light microscopy (A) and fluorescent microscopy (B) of ex vivo MPIO-labeled macrophages. (Scale bars: 50 μm.) (C) MRM at 11.7-Tesla using a Bruker AVANCE-DBX MRI instrument with in-plane resolution of 40 μm of gelatin phantoms containing isolated macrophages labeled with MPIO particles with different cell concentration.
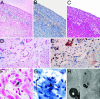
Fig. 5.
Histological examination of allograft hearts on POD 6 after in vivo MPIO-particle labeling. (A_–_C) Optical micrograph (×200 magnification) of three neighboring 5-μm tissue sections of a POD 6 allograft heart stained with Perl’s Prussian blue for iron (A, blue; with pink background counterstaining), anti-rat ED1 for macrophage (B, brown; with blue background counterstaining), and hematoxylin/eosin staining (C) for tissue integrity. (D and E) Optical micrograph (×400 magnification) of two neighboring 5-μm tissue sections of another POD 6 allograft heart stained with Perl’s Prussian blue for iron (D, blue; with pink background counterstaining), and anti-rat ED1 for macrophage (E, brown; with blue background counterstaining). (F and G) Partial view of optical micrograph (×400 magnification) of two neighboring 5-μm tissue sections of a POD 6 allograft heart stained with Perl’s Prussian blue for iron (F, blue; with pink background counterstaining) and anti-rat ED1 for macrophage (G, brown; with blue background counterstaining). (Scale bars: 75 μm.) (H) Electron micrograph of an allograft heart harvested on POD 6 after in vivo MPIO-particle labeling. (Inset) Enlarged area. (Scale bar: 100 nm.)
Fig. 6.
Double fluorescence for MPIO particles and ED1. Shown are fluorescence microscopy of Dragon green for MPIOs (A) and anti-ED1 (red) immunofluorescence microscopy for microphages of the same field of view (B). The boxes select three regions for overlaying double fluorescence. The overlay images are enlarged on the Right.
Fig. 7.
Temporal progression of ED1+ cell infiltration. Optical micrograph (×400 magnification) of anti-rat ED1+ immunohistochemical staining sections of allograft hearts obtained on POD 3 (A), POD 4 (B), POD 5 (C), and POD 6 (D). The ED1+ cells appear brown and the background is counterstained blue.
Similar articles
- Noninvasive evaluation of cardiac allograft rejection by cellular and functional cardiac magnetic resonance.
Wu YL, Ye Q, Sato K, Foley LM, Hitchens TK, Ho C. Wu YL, et al. JACC Cardiovasc Imaging. 2009 Jun;2(6):731-41. doi: 10.1016/j.jcmg.2009.01.013. JACC Cardiovasc Imaging. 2009. PMID: 19520344 Free PMC article. - Macrophage accumulation associated with rat cardiac allograft rejection detected by magnetic resonance imaging with ultrasmall superparamagnetic iron oxide particles.
Kanno S, Wu YJ, Lee PC, Dodd SJ, Williams M, Griffith BP, Ho C. Kanno S, et al. Circulation. 2001 Aug 21;104(8):934-8. doi: 10.1161/hc3401.093148. Circulation. 2001. PMID: 11514382 - Longitudinal tracking of recipient macrophages in a rat chronic cardiac allograft rejection model with noninvasive magnetic resonance imaging using micrometer-sized paramagnetic iron oxide particles.
Ye Q, Wu YL, Foley LM, Hitchens TK, Eytan DF, Shirwan H, Ho C. Ye Q, et al. Circulation. 2008 Jul 8;118(2):149-56. doi: 10.1161/CIRCULATIONAHA.107.746354. Circulation. 2008. PMID: 18591438 Free PMC article. - A non-invasive approach to detecting organ rejection by MRI: monitoring the accumulation of immune cells at the transplanted organ.
Ho C, Hitchens TK. Ho C, et al. Curr Pharm Biotechnol. 2004 Dec;5(6):551-66. doi: 10.2174/1389201043376535. Curr Pharm Biotechnol. 2004. PMID: 15579044 Review. - Non-invasive imaging for the diagnosis of acute rejection in transplantation: The next frontier.
Matar AJ, Crepeau RL, Duran-Struuck R. Matar AJ, et al. Transpl Immunol. 2021 Oct;68:101431. doi: 10.1016/j.trim.2021.101431. Epub 2021 Jun 19. Transpl Immunol. 2021. PMID: 34157374 Review.
Cited by
- Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death.
Kapralov AA, Yang Q, Dar HH, Tyurina YY, Anthonymuthu TS, Kim R, St Croix CM, Mikulska-Ruminska K, Liu B, Shrivastava IH, Tyurin VA, Ting HC, Wu YL, Gao Y, Shurin GV, Artyukhova MA, Ponomareva LA, Timashev PS, Domingues RM, Stoyanovsky DA, Greenberger JS, Mallampalli RK, Bahar I, Gabrilovich DI, Bayır H, Kagan VE. Kapralov AA, et al. Nat Chem Biol. 2020 Mar;16(3):278-290. doi: 10.1038/s41589-019-0462-8. Epub 2020 Feb 17. Nat Chem Biol. 2020. PMID: 32080625 Free PMC article. - Clinical Hepatocyte Transplantation: What Is Next?
Squires JE, Soltys KA, McKiernan P, Squires RH, Strom SC, Fox IJ, Soto-Gutierrez A. Squires JE, et al. Curr Transplant Rep. 2017 Dec;4(4):280-289. doi: 10.1007/s40472-017-0165-6. Epub 2017 Oct 14. Curr Transplant Rep. 2017. PMID: 29732274 Free PMC article. - Nanoparticle-Based Modulation and Monitoring of Antigen-Presenting Cells in Organ Transplantation.
Ochando J, Braza MS. Ochando J, et al. Front Immunol. 2017 Dec 22;8:1888. doi: 10.3389/fimmu.2017.01888. eCollection 2017. Front Immunol. 2017. PMID: 29312352 Free PMC article. Review. - Magnetosome-like ferrimagnetic iron oxide nanocubes for highly sensitive MRI of single cells and transplanted pancreatic islets.
Lee N, Kim H, Choi SH, Park M, Kim D, Kim HC, Choi Y, Lin S, Kim BH, Jung HS, Kim H, Park KS, Moon WK, Hyeon T. Lee N, et al. Proc Natl Acad Sci U S A. 2011 Feb 15;108(7):2662-7. doi: 10.1073/pnas.1016409108. Epub 2011 Jan 31. Proc Natl Acad Sci U S A. 2011. PMID: 21282616 Free PMC article. - Delivery of fluorescent probes using iron oxide particles as carriers enables in-vivo labeling of migrating neural precursors for magnetic resonance imaging and optical imaging.
Sumner JP, Conroy R, Shapiro EM, Moreland J, Koretsky AP. Sumner JP, et al. J Biomed Opt. 2007 Sep-Oct;12(5):051504. doi: 10.1117/1.2800294. J Biomed Opt. 2007. PMID: 17994868 Free PMC article.
References
- Cahill K. S., Gaidosh G., Huard J., Silver X., Byrne B. J., Walter G. A. Transplantation. 2004;78:1626–1633. - PubMed
- Bos C., Delmas Y., Desmouliere A., Solanilla A., Hauger O., Grosset C., Dubus I., Ivanovic Z., Rosenbaum J., Charbord P., et al. Radiology. 2004;233:781–789. - PubMed
- Bulte J. W., Duncan I. D., Frank J. A. J. Cereb. Blood Flow Metab. 2002;22:899–907. - PubMed
- Bulte J. W., Douglas T., Witwer B., Zhang S. C., Lewis B. K., van Gelderen P., Zywicke H., Duncan I. D., Frank J. A. Acad. Radiol. 2002;9:S332–S335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P41EB-001977/EB/NIBIB NIH HHS/United States
- R01 EB000318/EB/NIBIB NIH HHS/United States
- P41EB-00197/EB/NIBIB NIH HHS/United States
- F32HL-068423/HL/NHLBI NIH HHS/United States
- P41 EB001977/EB/NIBIB NIH HHS/United States
- F32 HL068423/HL/NHLBI NIH HHS/United States
- S10RR-15704/RR/NCRR NIH HHS/United States
- R01EB-00318/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical