Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes - PubMed (original) (raw)
Clinical Trial
Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes
Anny H Xiang et al. Diabetes. 2006 Feb.
Abstract
The Pioglitazone In Prevention Of Diabetes (PIPOD) study was conducted to evaluate beta-cell function, insulin resistance, and the incidence of diabetes during treatment with pioglitazone in Hispanic women with prior gestational diabetes who had completed participation in the Troglitazone In Prevention Of Diabetes (TRIPOD) study. Women who completed the TRIPOD study were offered participation in the PIPOD study for a planned 3 years of drug treatment and 6 months of postdrug washout. Oral glucose tolerance tests were performed annually on pioglitazone and at the end of the postdrug washout. Intravenous glucose tolerance tests (IVGTTs) for assessment of insulin sensitivity and beta-cell function were conducted at baseline, after 1 year on pioglitazone, and at the end of the postdrug washout. Of 95 women who were not diabetic at the end of the TRIPOD study, 89 enrolled in the PIPOD study, 86 completed at least one follow-up visit, and 65 completed all study visits, including the postdrug tests. Comparison of changes in beta-cell compensation for insulin resistance across the TRIPOD and PIPOD studies revealed that pioglitazone stopped the decline in beta-cell function that occurred during placebo treatment in the TRIPOD study and maintained the stability of beta-cell function that had occurred during troglitazone treatment in the TRIPOD study. The risk of diabetes, which occurred at an average rate of 4.6% per year, was lowest in women with the largest reduction in total IVGTT insulin area after 1 year of treatment. The similarity of findings between the PIPOD and TRIPOD studies support a class effect of thiazolidinedione drugs to enhance insulin sensitivity, reduce insulin secretory demands, and preserve pancreatic beta-cell function, all in association with a relatively low rate of type 2 diabetes, in Hispanic women with prior gestational diabetes.
Figures
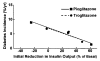
FIG. 1
Relationship between initial fractional reduction in IVGTT insulin area and corresponding diabetes incidence rates in the troglitazone arm of the TRIPOD study (•) and in the PIPOD study (▪). Insulin output was assessed as the total area under the insulin curve during IVGTTs. Reductions in output were calculated between enrollment into each study and the initial on-treatment IVGTT, which occurred after 3 months in the TRIPOD study and after 1 year in the PIPOD study. Symbols represent the low, middle, and high tertile of change in each study. Lines represent best linear fits of data for each study.
Similar articles
- Effect of pioglitazone on progression of subclinical atherosclerosis in non-diabetic premenopausal Hispanic women with prior gestational diabetes.
Xiang AH, Hodis HN, Kawakubo M, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Liu CR, Liu CH, Buchanan TA. Xiang AH, et al. Atherosclerosis. 2008 Jul;199(1):207-14. doi: 10.1016/j.atherosclerosis.2007.10.016. Epub 2007 Dec 4. Atherosclerosis. 2008. PMID: 18054942 Free PMC article. Clinical Trial. - Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women.
Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP. Buchanan TA, et al. Diabetes. 2002 Sep;51(9):2796-803. doi: 10.2337/diabetes.51.9.2796. Diabetes. 2002. PMID: 12196473 Clinical Trial. - Effect of troglitazone on insulin sensitivity and pancreatic beta-cell function in women at high risk for NIDDM.
Berkowitz K, Peters R, Kjos SL, Goico J, Marroquin A, Dunn ME, Xiang A, Azen S, Buchanan TA. Berkowitz K, et al. Diabetes. 1996 Nov;45(11):1572-9. doi: 10.2337/diab.45.11.1572. Diabetes. 1996. PMID: 8866563 Clinical Trial. - Pioglitazone: a review of its use in type 2 diabetes mellitus.
Waugh J, Keating GM, Plosker GL, Easthope S, Robinson DM. Waugh J, et al. Drugs. 2006;66(1):85-109. doi: 10.2165/00003495-200666010-00005. Drugs. 2006. PMID: 16398569 Review. - Pioglitazone and sulfonylureas: effectively treating type 2 diabetes.
Hanefeld M. Hanefeld M. Int J Clin Pract Suppl. 2007 Jun;61(153):20-7. doi: 10.1111/j.1742-1241.2007.01361.x. Int J Clin Pract Suppl. 2007. PMID: 17594390 Free PMC article. Review.
Cited by
- Gestational Diabetes Mellitus and Subsequent Risks of Diabetes and Cardiovascular Diseases: the Life Course Perspective and Implications of Racial Disparities.
Chen L, Zhu Y. Chen L, et al. Curr Diab Rep. 2024 Nov;24(11):244-255. doi: 10.1007/s11892-024-01552-4. Epub 2024 Sep 4. Curr Diab Rep. 2024. PMID: 39230861 Review. - Pancreatic beta-cell mass and function and therapeutic implications of using antidiabetic medications in type 2 diabetes.
Moon JH, Choe HJ, Lim S. Moon JH, et al. J Diabetes Investig. 2024 Jun;15(6):669-683. doi: 10.1111/jdi.14221. Epub 2024 Apr 27. J Diabetes Investig. 2024. PMID: 38676410 Free PMC article. Review. - Beta-cell function in type 2 diabetes (T2DM): Can it be preserved or enhanced?
Sayyed Kassem L, Rajpal A, Barreiro MV, Ismail-Beigi F. Sayyed Kassem L, et al. J Diabetes. 2023 Oct;15(10):817-837. doi: 10.1111/1753-0407.13446. Epub 2023 Jul 31. J Diabetes. 2023. PMID: 37522521 Free PMC article. Review. - Genetics and Epigenetics: Implications for the Life Course of Gestational Diabetes.
Lowe WL Jr. Lowe WL Jr. Int J Mol Sci. 2023 Mar 23;24(7):6047. doi: 10.3390/ijms24076047. Int J Mol Sci. 2023. PMID: 37047019 Free PMC article. Review.
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus (Position Statement) Diabetes Care. 2004;27 (Suppl 1):S5–S10. - PubMed
- Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992;340:925–929. - PubMed
- Haffner SM, Miettinen H, Gaskill SP, Stern MP. Decreased insulin secretion and increased insulin resistance are independently related to the 7-year risk of NIDDM in Mexican Americans. Diabetes. 1995;44:1386–1391. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical