Derlin-2 and Derlin-3 are regulated by the mammalian unfolded protein response and are required for ER-associated degradation - PubMed (original) (raw)
Derlin-2 and Derlin-3 are regulated by the mammalian unfolded protein response and are required for ER-associated degradation
Yukako Oda et al. J Cell Biol. 2006.
Abstract
Proteins that are unfolded or misfolded in the endoplasmic reticulum (ER) must be refolded or degraded to maintain the homeostasis of the ER. Components of both productive folding and ER-associated degradation (ERAD) mechanisms are known to be up-regulated by the unfolded protein response (UPR). We describe two novel components of mammalian ERAD, Derlin-2 and -3, which show weak homology to Der1p, a transmembrane protein involved in yeast ERAD. Both Derlin-2 and -3 are up-regulated by the UPR, and at least Derlin-2 is a target of the IRE1 branch of the response, which is known to up-regulate ER degradation enhancing alpha-mannosidase-like protein (EDEM) and EDEM2, receptor-like molecules for misfolded glycoprotein. Overexpression of Derlin-2 or -3 accelerated degradation of misfolded glycoprotein, whereas their knockdown blocked degradation. Derlin-2 and -3 are associated with EDEM and p97, a cytosolic ATPase responsible for extraction of ERAD substrates. These findings indicate that Derlin-2 and -3 provide the missing link between EDEM and p97 in the process of degrading misfolded glycoproteins.
Figures
Figure 1.
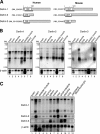
Identification of Derlin-2 and -3. (A) The levels of CGI-101, BiP, and β-actin mRNA are determined by microarray analysis in HeLa cells treated with or without 2 μg/ml tunicamycin for 8 h. Fold induction was determined, with the means from six independent experiments presented with SDs (error bars). CGI-101 is identical to Derlin-2. (B) Hydropathy plots of human Derlin-2 (CGI-101), -3 (FLJ43842), and -1 are shown. Hydrophobicity and hydrophilicity (expressed by positive and negative numbers, respectively) of the amino acid sequences of three human Derlins were obtained according to the method of Kyte and Doolittle (1982). Black bars mark hydrophobic regions that span the membrane. (C) Amino acid sequence alignment of human Derlin-2, Derlin-3 tv1, Derlin-3 tv2, Derlin-1, and yeast Der1p is shown. Identical amino acids are indicated by white letters in black boxes. Two transcriptional variants for Derlin-3 are deposited in the data bank. Derlin-3 tv1 lacks the 30 COOH-terminal amino acids present in Derlin-3 tv2.
Figure 2.
Structure and tissue distribution of Derlin-2 and -3 mRNA. (A) Schematic structures of transcripts deposited in the data bank for human (available from GenBank/EMBL/DDBJ under accession no. NM_024295) and mouse (NM_024207) Derlin-1, human (NM_016041) and mouse (NM_033562) Derlin-2, and human (NM_001002862) and mouse (NM_024440) Derlin-3 are shown. Black underlines indicate the respective region of cDNA probe used for Northern blot analysis. (B) A nylon membrane onto which ∼2 μg each of poly A+ RNA prepared from eight human tissues was blotted after separation through gel electrophoresis (Human MTN blot) was hybridized with a DIG-labeled cDNA probe specific to human Derlin-1, Derlin-2, Derlin-3, or β-actin. Migration positions of molecular weight markers are indicated on the left of each panel. (C) A second nylon membrane onto which ∼1 μg each of poly A+ RNA prepared from 12 human tissues was blotted after separation through gel electrophoresis (Human 12-lane MTN blot) was hybridized as in B.
Figure 3.
Involvement of the IRE1–XBP1 pathway in the induction of Derlin-1 and -2 in response to ER stress. (A) IRE1α+/+, IRE1α−/−, XBP1+/+, and XBP1−/− MEFs were treated with 10 μg/ml tunicamycin (Tm) for the indicated periods. Total RNAs were isolated and analyzed by Northern blot hybridization using a DIG-labeled cDNA probe specific to mouse Derlin-2, Derlin-1, EDEM, BiP, or GAPDH. Closed and open arrowheads indicate the migration positions of 28S ribosomal RNA (4.7 kb) and 18S ribosomal RNA (1.9 kb), respectively. (B) 293T or HeLa cells were treated with 2 μg/ml tunicamycin (Tm) for the indicated periods. Total RNAs were analyzed as in A using a DIG-labeled cDNA probe specific to human Derlin-2, Derlin-3, EDEM, BiP, or GAPDH.
Figure 4.
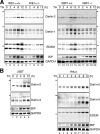
Characterization of Derlin-2 and -3. (A) HeLa cells were trans-fected with plasmid to express each Derlin tagged with the c-myc epitope at the respective NH2 terminus. Transfected cells were fixed and stained with anti-myc and anti-Sec61β antibodies. (B) HEK293 cells were trans-fected with plasmid to express each Derlin tagged with the c-myc epitope at the respective COOH terminus. Postnuclear supernatant of transfected cells was incubated with increasing amounts of trypsin (0, 4, 8, and 16 μg for lane 1, 2, 3, and 4, respectively) for 15 min at 4°C. Immunoblotting analysis of the samples was performed using anti-myc, anti–NH2 terminus of calnexin (CNX[N]), anti–COOH terminus of calnexin (CNX[C]), and anti-calreticulin (CRT) antibodies. (C) HEK293 cells were transfected with plasmid to express Derlin-2 (a) or Derlin-3 tv1 or tv2 (b) tagged with the c-myc epitope at either the NH2 (N) or COOH (C) terminus. 36 h later, transfected cells were pulse labeled with 35S-methionine and cysteine for 15 min and then chased for the indicated periods. Cells were lysed with buffer containing 1% NP-40 and subjected to immunoprecipitation analysis using anti-myc antibody.
Figure 5.
Effects of overexpression of Derlin-2 and -3 on degradation of NHK and NHK(QQQ). (A and B) HEK293 cells were mock-transfected or transfected with plasmid to express Derlin-2 or Derlin-3 tv2 tagged with the c-myc epitope at the respective NH2 terminus together with plasmid to express NHK (A) or NHK(QQQ) (B). Transfected cells were pulse-chased and subjected to immunoprecipitation analysis using anti–α1-PI antibody as in Fig. 4 C. Migration positions of NHK, NHK(QQQ), Derlin-2, and Derlin-3 are indicated. The radioactivity of each NHK band was determined and expressed as relative to the summation of radioactivity of nine NHK bands obtained in each experiment. The relative radioactivity of each band was normalized with the value at chase period 0 h. The means from three independent experiments with SDs (error bars) are plotted against the chase period (bottom).
Figure 6.
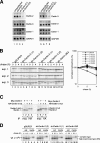
Effects of knockdown of Derlin-2 and -3 on degradation of NHK. (A) HEK293 cells were untransfected or transfected with the shRNA vector pSUPER alone or pSUPER carrying a sequence corresponding to a part of Derlin-2 or -3. Total RNAs were isolated 64 h after transfection and analyzed by Northern blot hybridization using a DIG-labeled cDNA probe specific to human Derlin-1, Derlin-2, Derlin-3, or GAPDH. (B) HEK293 cells were transfected with pSUPER alone or pSUPER carrying a sequence corresponding to a part of Derlin-2, Derlin-3, or both together with plasmid to express NHK. Pulse-chase and subsequent immunoprecipitation were performed as in Fig. 5 A 64 h after transfection. The results of three independent experiments are shown (left). Normalized radioactivity of each NHK band was determined and is presented as in Fig. 5 A (right). Error bars depict means ± SD. (C) HEK293 cells were transfected with (+) or without (−) plasmid to express Derlin-1 or -2 tagged with the c-myc epitope at the respective NH2 terminus with (+) or without (−) plasmid to express Derlin-3 tv2 tagged with the HA epitope at the NH2 terminus. 36 h later, transfected cells were labeled with 35S-methionine and cysteine for 2 h, lysed with buffer containing 1% NP-40, and subjected to immunoprecipitation analysis using anti-myc or anti-HA antibody as indicated. Migration positions of Derlin-1, Derlin-2, and Derlin-3 tv2 are marked. (D) HEK293 cells were transfected with pSUPER alone or pSUPER derivatives as in B together with plasmid to express the wild-type (wt) α1-PI. Pulse-chase and subsequent immunoprecipitation from cell lysate as well as from media were performed as in B. Migration positions of high-mannose and complex wild-type α1-PI are indicated.
Figure 7.
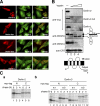
Association of Derlin-2 and -3 with p97, EDEM, and NHK. (A) HEK293 cells were mock-transfected or transfected with plasmid to express each Derlin tagged with the c-myc epitope at the respective COOH terminus alone (a) or with plasmid to express FLAG-tagged p97 (b). HEK293 cells were also transfected with plasmid to express each Derlin tagged with the c-myc epitope at the respective NH2 terminus (c). 24 h later, transfected cells were labeled with 35S-methionine and cysteine for 1 h, lysed with buffer containing 1% NP-40, and subjected to immunoprecipitation analysis using anti-myc or anti-p97 antibody as indicated. Migration positions of endogenous and FLAG-tagged p97 are marked. The asterisk shows a nonspecific band. The short open arrowhead indicates p97 coimmunoprecipitated with Derlins, whereas long open arrowheads indicate Derlins coimmunoprecipitated with p97. (B, a) HEK293 cells were mock-transfected or transfected with plasmid to express each Derlin tagged with the c-myc epitope at the respective NH2 terminus together with plasmid to express HA-tagged EDEM. 24 h later, transfected cells were labeled with 35S-methionine and cysteine for 2 h, lysed, and subjected to immunoprecipitation analysis using anti-myc or anti-HA antibody as indicated. The migration position of EDEM is marked. The asterisk shows a nonspecific band. The short open arrowhead indicates EDEM coimmunoprecipitated with Derlins, whereas long open arrowheads indicate Derlins coimmunoprecipitated with EDEM. (b and c) HEK293 cells were transfected with (+) or without (−) plasmid to express HA-tagged EDEM with or without plasmid to express Derlin-1 or -2 tagged with the c-myc epitope at the respective NH2 terminus. Transfected cells pulse labeled and then lysed as in a were subjected to immunoprecipitation analysis using anti-HA or anti-p97 antibody as indicated. (c) The amount of Derlin-1 expression plasmid to transfect HEK293 cells was twice that of Derlin-2 expression plasmid. Migration positions of p97, EDEM, and Derlins coimmunoprecipitated with EDEM or p97 are indicated. (C) HEK293 cells were transfected with (+) plasmid to express NHK and with or without plasmid to express Derlin-1 or -2 tagged with the c-myc epitope at the respective NH2 terminus. Transfected cells were pulse labeled and then lysed as in panel a. Equal amounts of cell lysate were subjected to immunoprecipitation analysis using anti–α1-PI, anti-p97, or anti-myc antibody as indicated. Migration positions of p97, NHK, and Derlins are indicated.
Figure 8.
Effects of knockdown of Derlin-1 on degradation of NHK. (A) HEK293 cells were untransfected or transfected with pSUPER alone or pSUPER carrying a sequence corresponding to a part of Derlin-1, -2, or -3. 64 h later, transfected cells were subjected to immunoblotting analysis using antibody against Derlin-1, Derlin-2, or GAPDH. (B) HEK293 cells were transfected with pSUPER alone or pSUPER carrying a sequence corresponding to a part of Derlin-1 or -3 together with plasmid to express NHK. 64 h later, transfected cells were pulse-chased, lysed, and subjected to immunoprecipitation as in Fig. 6 B (left). The results of three independent experiments are shown. Normalized radioactivity of each NHK band was also determined and is presented as in Fig. 5 A (right). Error bars depict mean ± SD.
Similar articles
- Multiprotein complexes that link dislocation, ubiquitination, and extraction of misfolded proteins from the endoplasmic reticulum membrane.
Lilley BN, Ploegh HL. Lilley BN, et al. Proc Natl Acad Sci U S A. 2005 Oct 4;102(40):14296-301. doi: 10.1073/pnas.0505014102. Epub 2005 Sep 26. Proc Natl Acad Sci U S A. 2005. PMID: 16186509 Free PMC article. - Yeast Derlin Dfm1 interacts with Cdc48 and functions in ER homeostasis.
Sato BK, Hampton RY. Sato BK, et al. Yeast. 2006 Oct-Nov;23(14-15):1053-64. doi: 10.1002/yea.1407. Yeast. 2006. PMID: 17083136 - A novel ER alpha-mannosidase-like protein accelerates ER-associated degradation.
Hosokawa N, Wada I, Hasegawa K, Yorihuzi T, Tremblay LO, Herscovics A, Nagata K. Hosokawa N, et al. EMBO Rep. 2001 May;2(5):415-22. doi: 10.1093/embo-reports/kve084. EMBO Rep. 2001. PMID: 11375934 Free PMC article. - Mif1: a missing link between the unfolded protein response pathway and ER-associated protein degradation?
van Laar T, van der Eb AJ, Terleth C. van Laar T, et al. Curr Protein Pept Sci. 2001 Jun;2(2):169-90. doi: 10.2174/1389203013381189. Curr Protein Pept Sci. 2001. PMID: 12370023 Review. - The Role of Lectin-Carbohydrate Interactions in the Regulation of ER-Associated Protein Degradation.
Słomińska-Wojewódzka M, Sandvig K. Słomińska-Wojewódzka M, et al. Molecules. 2015 May 27;20(6):9816-46. doi: 10.3390/molecules20069816. Molecules. 2015. PMID: 26023941 Free PMC article. Review.
Cited by
- Identification of new protein interactions between dengue fever virus and its hosts, human and mosquito.
Mairiang D, Zhang H, Sodja A, Murali T, Suriyaphol P, Malasit P, Limjindaporn T, Finley RL Jr. Mairiang D, et al. PLoS One. 2013;8(1):e53535. doi: 10.1371/journal.pone.0053535. Epub 2013 Jan 11. PLoS One. 2013. PMID: 23326450 Free PMC article. - Hepatic inactivation of murine Surf4 results in marked reduction in plasma cholesterol.
Tang VT, McCormick J, Xu B, Wang Y, Fang H, Wang X, Siemieniak D, Khoriaty R, Emmer BT, Chen XW, Ginsburg D. Tang VT, et al. Elife. 2022 Oct 4;11:e82269. doi: 10.7554/eLife.82269. Elife. 2022. PMID: 36193893 Free PMC article. - Aerobic exercise training rescues cardiac protein quality control and blunts endoplasmic reticulum stress in heart failure rats.
Bozi LH, Jannig PR, Rolim N, Voltarelli VA, Dourado PM, Wisløff U, Brum PC. Bozi LH, et al. J Cell Mol Med. 2016 Nov;20(11):2208-2212. doi: 10.1111/jcmm.12894. Epub 2016 Jun 16. J Cell Mol Med. 2016. PMID: 27305869 Free PMC article. - Glycoprotein misfolding in the endoplasmic reticulum: identification of released oligosaccharides reveals a second ER-associated degradation pathway for Golgi-retrieved proteins.
Alonzi DS, Kukushkin NV, Allman SA, Hakki Z, Williams SJ, Pierce L, Dwek RA, Butters TD. Alonzi DS, et al. Cell Mol Life Sci. 2013 Aug;70(15):2799-814. doi: 10.1007/s00018-013-1304-6. Epub 2013 Mar 16. Cell Mol Life Sci. 2013. PMID: 23503623 Free PMC article. - ER Stress Proteins in Autoimmune and Inflammatory Diseases.
Morito D, Nagata K. Morito D, et al. Front Immunol. 2012 Mar 15;3:48. doi: 10.3389/fimmu.2012.00048. eCollection 2012. Front Immunol. 2012. PMID: 22566930 Free PMC article.
References
- Brummelkamp, T.R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science. 296:550–553. - PubMed
- Dai, R.M., and C.C. Li. 2001. Valosin-containing protein is a multi-ubiquitin chain-targeting factor required in ubiquitin-proteasome degradation. Nat. Cell Biol. 3:740–744. - PubMed
- Gething, M.J., and J. Sambrook. 1992. Protein folding in the cell. Nature. 355:33–45. - PubMed
- Harding, H.P., M. Calfon, F. Urano, I. Novoa, and D. Ron. 2002. Transcriptional and translational control in the mammalian unfolded protein response. Annu. Rev. Cell Dev. Biol. 18:575–599. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases