Neuronal cotransport of glycine receptor and the scaffold protein gephyrin - PubMed (original) (raw)
Neuronal cotransport of glycine receptor and the scaffold protein gephyrin
Christoph Maas et al. J Cell Biol. 2006.
Abstract
The dynamics of postsynaptic receptor scaffold formation and remodeling at inhibitory synapses remain largely unknown. Gephyrin, which is a multimeric scaffold protein, interacts with cytoskeletal elements and stabilizes glycine receptors (GlyRs) and individual subtypes of gamma-aminobutyric acid A receptors at inhibitory postsynaptic sites. We report intracellular mobility of gephyrin transports packets over time. Gephyrin units enter and exit active synapses within several minutes. In addition to previous reports of GlyR-gephyrin interactions at plasma membranes, we show cosedimentation and coimmunoprecipitation of both proteins from vesicular fractions. Moreover, GlyR and gephyrin are cotransported within neuronal dendrites and further coimmunoprecipitate and colocalize with the dynein motor complex. As a result, the blockade of dynein function or dynein-gephyrin interaction, as well as the depolymerization of microtubules, interferes with retrograde gephyrin recruitment. Our data suggest a GlyR-gephyrin-dynein transport complex and support the concept that gephyrin-motor interactions contribute to the dynamic and activity-dependent rearrangement of postsynaptic GlyRs, a process thought to underlie the regulation of synaptic strength.
Figures
Figure 1.
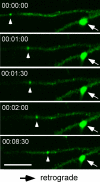
Gephyrin transport units are rapidly recruited in dendrites of cultured hippocampal neurons. A small gephyrin particle (arrowhead) moved in a retrograde direction toward a dendritic branch point over time. In contrast, a larger gephyrin aggregate on the other branch (arrow) was immobile. Bar, 5 μm.
Figure 2.
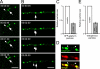
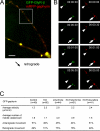
Recruitment of GFP–gephyrin transport units to and from sites of immobile gephyrin clusters. Images were acquired every 30 or 45 s. (A) Small particle recruitment (arrowhead) toward a larger immobile gephyrin cluster (arrow). (B) A small cluster (arrowhead) left an immobile site of gephyrin autofluorescence (arrow) over time. Note that the shift in focus is caused by temperature variations during image acquisition. (C) Relative particle size of immobile and mobile GFP–gephyrin puncta. (D) Detection of two populations of endogenous gephyrin clusters within dendrites with a significant size difference. Large endogenous gephyrin clusters (green) colocalized with the presynaptic marker VIAAT (red), as represented in the merged image (yellow). In contrast, small clusters, which were frequently mobile in time-lapse experiments, did not colocalize with presynaptic terminal boutons (arrowheads). (E) Relative particle size of synaptic and nonsynaptic endogenous gephyrin puncta. Error bars represent size variations of mobile clusters, as compared with immobile clusters. Bars: (A and B) 5 μm; (D) 2 μm.
Figure 3.
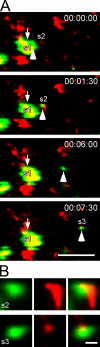
Recruitment of gephyrin transport packets from and to active synapses. Cultures were supplemented with FM4-64 dye (red) to visualize active presynaptic terminal boutons. Pre- and postsynaptic appositions are depicted as s1–s3. Colocalization of GFP–gephyrin– (green) and FM4-64–positive terminals (red) is represented in yellow. (A) A gephyrin transport packet (arrowhead), which emerged from synapse s1 (arrow), rapidly merged with/emerged from other synapses. Over a total time period of ∼7 min, the mobile gephyrin packet colocalized with different active terminal boutons. Note that loss or appearance of individual red fluorescent puncta is because of the flexibility of individual axon terminals within the culture system and subsequent shift in focus during image acquisition. (B) Magnification of synapses s2 and s3 from A. Bars: (A) 5 μm; (B) 0.2 μm.
Figure 4.
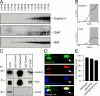
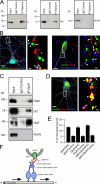
Association of gephyrin and GlyR at cytoplasmic vesicle-rich cell compartments. (A) Cosedimentation of gephyrin and GlyR upon sucrose gradient centrifugation of 160,000 g vesicle-enriched pellets. NSF detection was used as a loading control. (B) The majority of gephyrin and GlyR immunoreactivity is detected above the 1.0-M fraction. (C) Coimmunoprecipitation on rat brain cytoplasmic vesicle–enriched cell fractions using both gephyrin- and GlyR-specific antibodies. Beads coupled with antibody, but not with control IgG, retain gephyrin and GlyR, but not GluR2. (D) Triple detection of endogenous gephyrin, GlyR, and the synaptic marker synaptophysin in dendrites of cultured hippocampal neurons. In contrast to synaptic sites (white), individual small-size nonsynaptic puncta display GlyR–gephyrin colocalization (yellow; arrows). These particles represent putative molecules in transit. (E) Quantitative evaluation of colocalized puncta. Error bars represent variations between individual experiments. Bar, 1.5 μm.
Figure 5.
Cotransport of gephyrin and GlyRβ subunits in cultured hippocampal neurons. (A) Neuronal coexpression of GFP–GlyRβ and mRFP–gephyrin. Both fusion proteins cluster and colocalize in dendrites. (B) Time-lapse video microscopy revealed cotransport of both puncta over time. Two particles in each fluorescent channel (arrowheads) are recruited in the retrograde direction toward the dendritic branch point shown in A. An immobile cluster of GlyR–gephyrin coimmunoreactivity is indicated by arrows. (C) GFP–gephyrin transport particle characteristics upon 10 mM KCl-induced neuronal depolarization and/or application of the GlyR and GABAAR antagonists strychnine and bicuculline, respectively. Velocity changes upon KCl or strychnine application and the shift toward retrograde movement upon strychnine application are significant (P < 0.001), as compared with control values. Bar, 5μm.
Figure 6.
Formation of a GlyR–gephyrin–dynein triple complex. (A) Coimmunoprecipitation of gephyrin and the 74-kD DIC, which is retained from beads coupled with monoclonal gephyrin antibody, but not coupled with control IgG. GluR2 detection serves as a negative control. (B) Colocalization of endogenous gephyrin or GlyR (green) with DHC (red) and synapse markers (blue) in neuronal dendrites. Magnifications of insets are shown to the right of the images. Note that putative molecules in transit locate at nonsynaptic sites (arrows). Synaptic sites are marked by arrowheads. (C) Triple coimmunoprecipitation experiment. Beads coupled with GlyR-specific antibody, but not coupled with control IgG, retain GlyR, gephyrin, and DIC, but not GluR2. (D) Immunocytochemical detection of endogenous GlyR, gephyrin, and DHC. Note that triple complex formations (white, arrows) are putative transport molecules. Large yellow clusters represent GlyR–gephyrin colocalization at putative synapses (arrowheads). Magnification of the inset is shown to the right of the image. (E) Quantitative evaluation of gephyrin, GlyR, and DHC colocalization experiments shown in B and D. Values are significantly (P < 0.001) above colocalization values obtained with the unrelated motor protein KIF1B. (F) Schematic representation of the GlyR–gephyrin–dynein transport complex. Bars: (B and D) 5 μm; (B and D, magnifications) 0.5 μm.
Figure 7.
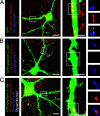
Inhibition of dynein-mediated transport in neurons. The blockade of dynein motor function prevents the dominant-negative–induced loss of synaptic gephyrin clusters in cultured hippocampal neurons. (A–C) Boxed regions are shown at higher magnification. Yellow indicates fluorescent overlap of endogenous gephyrin (red) and GFP or GFP-fusion protein (green). The magenta color represents fluorescent overlap of gephyrin (red) and synaptic sites (blue). Red aggregates outside the fluorescent cell (green) represent gephyrin clusters of nontransfected cells within the culture. (A) Control condition visualizing synaptic gephyrin clusters in GFP-expressing neurons. (B) Upon overexpression of a dominant-negative construct fused to GFP (GFP–gephyrin 2–188), preexisting gephyrin clusters are lost in neurite processes. (C) Dynamitin-induced inhibition of dynein motor function prevents loss of preexisting gephyrin clusters in the presence of the dominant-negative construct described in B. Remaining gephyrin clusters are localized at synaptic sites. Synapses are represented by synaptophysin immunoreactivity. Bars: (A–C) 15 μm; (A–C, magnifications) 5 μm.
Figure 8.
Competition of dynein-mediated gephyrin transport by overexpression of the isolated DLC-binding motif of gephyrin. (A) Schematic representation of the gephyrin domain structure (Sola et al., 2004). The DLC-binding motif, which is located between gephyrin residues 181–243 (Fuhrmann et al., 2002), is fused to mRFP (mRFP–gephyrin 181–243). (B) Singly expressed mRFP–gephyrin 181–243 fusion protein (arrowhead) is retrogradely transported toward a dendritic branch point (arrow) in cultured hippocampal neurons over time. (C) Dual-channel time-lapse recording of cultured hippocampal neurons expressing GFP–gephyrin and mRFP–gephyrin 181–243. Localization of GFP–gephyrin clusters at distal dendrites (crossed arrows), which depends on anterograde transport, is normal, whereas retrograde transport of GFP–gephyrin is inhibited. In contrast, retrograde transport of mRFP–gephyrin 181–243 is frequently observed. Note that at several days of expression, GFP–gephyrin scaffold turnover is blocked in both directions, whereas mRFP–gephyrin 181–243 remains mobile. The boxed region is shown at higher magnification. Arrows indicate immobile puncta, arrowheads indicate three mobile particles (1, 2, and 3) that subsequently move toward the cell body. Bars: (B) 3 μm; (C) 5 μm.
Similar articles
- A gephyrin-related mechanism restraining glycine receptor anchoring at GABAergic synapses.
Meier J, Grantyn R. Meier J, et al. J Neurosci. 2004 Feb 11;24(6):1398-405. doi: 10.1523/JNEUROSCI.4260-03.2004. J Neurosci. 2004. PMID: 14960612 Free PMC article. - Intracellular association of glycine receptor with gephyrin increases its plasma membrane accumulation rate.
Hanus C, Vannier C, Triller A. Hanus C, et al. J Neurosci. 2004 Feb 4;24(5):1119-28. doi: 10.1523/JNEUROSCI.4380-03.2004. J Neurosci. 2004. PMID: 14762130 Free PMC article. - Gephyrin oligomerization controls GlyR mobility and synaptic clustering.
Calamai M, Specht CG, Heller J, Alcor D, Machado P, Vannier C, Triller A. Calamai M, et al. J Neurosci. 2009 Jun 17;29(24):7639-48. doi: 10.1523/JNEUROSCI.5711-08.2009. J Neurosci. 2009. PMID: 19535575 Free PMC article. - Gephyrin: a master regulator of neuronal function?
Tyagarajan SK, Fritschy JM. Tyagarajan SK, et al. Nat Rev Neurosci. 2014 Mar;15(3):141-56. doi: 10.1038/nrn3670. Nat Rev Neurosci. 2014. PMID: 24552784 Review. - Gephyrin and the regulation of synaptic strength and dynamics at glycinergic inhibitory synapses.
Alvarez FJ. Alvarez FJ. Brain Res Bull. 2017 Mar;129:50-65. doi: 10.1016/j.brainresbull.2016.09.003. Epub 2016 Sep 6. Brain Res Bull. 2017. PMID: 27612963 Review.
Cited by
- The ataxia (axJ) mutation causes abnormal GABAA receptor turnover in mice.
Lappe-Siefke C, Loebrich S, Hevers W, Waidmann OB, Schweizer M, Fehr S, Fritschy JM, Dikic I, Eilers J, Wilson SM, Kneussel M. Lappe-Siefke C, et al. PLoS Genet. 2009 Sep;5(9):e1000631. doi: 10.1371/journal.pgen.1000631. Epub 2009 Sep 4. PLoS Genet. 2009. PMID: 19759851 Free PMC article. - Postnatal development of glycine receptor subunits α1, α2, α3, and β immunoreactivity in multiple brain stem respiratory-related nuclear groups of the rat.
Liu Q, Wong-Riley MT. Liu Q, et al. Brain Res. 2013 Nov 13;1538:1-16. doi: 10.1016/j.brainres.2013.09.028. Epub 2013 Sep 27. Brain Res. 2013. PMID: 24080401 Free PMC article. - Alpha subunit-dependent glycine receptor clustering and regulation of synaptic receptor numbers.
Patrizio A, Renner M, Pizzarelli R, Triller A, Specht CG. Patrizio A, et al. Sci Rep. 2017 Sep 7;7(1):10899. doi: 10.1038/s41598-017-11264-3. Sci Rep. 2017. PMID: 28883437 Free PMC article. - Cellular transport and membrane dynamics of the glycine receptor.
Dumoulin A, Triller A, Kneussel M. Dumoulin A, et al. Front Mol Neurosci. 2010 Feb 5;2:28. doi: 10.3389/neuro.02.028.2009. eCollection 2009. Front Mol Neurosci. 2010. PMID: 20161805 Free PMC article. - Gephyrin: a key regulatory protein of inhibitory synapses and beyond.
Groeneweg FL, Trattnig C, Kuhse J, Nawrotzki RA, Kirsch J. Groeneweg FL, et al. Histochem Cell Biol. 2018 Nov;150(5):489-508. doi: 10.1007/s00418-018-1725-2. Epub 2018 Sep 27. Histochem Cell Biol. 2018. PMID: 30264265 Review.
References
- Ackermann, M., and A. Matus. 2003. Activity-induced targeting of profilin and stabilization of dendritic spine morphology. Nat. Neurosci. 6:1194–1200. - PubMed
- Choquet, D., and A. Triller. 2003. The role of receptor diffusion in the organization of the postsynaptic membrane. Nat. Rev. Neurosci. 4:251–265. - PubMed
- Danglot, L., P. Rostaing, A. Triller, and A. Bessis. 2004. Morphologically identified glycinergic synapses in the hippocampus. Mol. Cell. Neurosci. 27:394–403. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials