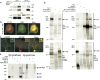Alzheimer precursor protein interaction with the Nogo-66 receptor reduces amyloid-beta plaque deposition - PubMed (original) (raw)
Alzheimer precursor protein interaction with the Nogo-66 receptor reduces amyloid-beta plaque deposition
James H Park et al. J Neurosci. 2006.
Abstract
Pathophysiologic hypotheses for Alzheimer's disease (AD) are centered on the role of the amyloid plaque Abeta peptide and the mechanism of its derivation from the amyloid precursor protein (APP). As part of the disease process, an aberrant axonal sprouting response is known to occur near Abeta deposits. A Nogo to Nogo-66 receptor (NgR) pathway contributes to determining the ability of adult CNS axons to extend after traumatic injuries. Here, we consider the potential role of NgR mechanisms in AD. Both Nogo and NgR are mislocalized in AD brain samples. APP physically associates with the NgR. Overexpression of NgR decreases Abeta production in neuroblastoma culture, and targeted disruption of NgR expression increases transgenic mouse brain Abeta levels, Abeta plaque deposition, and dystrophic neurites. Infusion of a soluble NgR fragment reduces Abeta levels, amyloid plaque deposits, and dystrophic neurites in a mouse transgenic AD model. Changes in NgR level produce parallel changes in secreted APPalpha and Abeta, implicating NgR as a blocker of secretase processing of APP. The NgR provides a novel site for modifying the course of AD and highlights the role of axonal dysfunction in the disease.
Figures
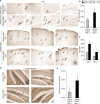
Figure 1.
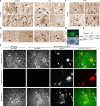
Altered Nogo and NgR localization in human AD brain.a–c, Human brain samples from age-matched control samples, AD, or diffuse Lewy body dementia (DLB) were processed for anti-NgR or anti-Nogo-A immunohistology as indicated. All samples are from the hippocampus. Note the reduced cellular NgR localization in AD and the enhanced cellular Nogo localization in the AD samples (arrowheads, cellular staining). Some amyloid plaques are NgR immunoreactive (arrows). d, The colocalization of NgR and Aβ in plaques is shown in higher magnification at the bottom right (arrows). e, Anti-NgR immunoblot demonstrates the specificity of the anti-NgR antibody and the unchanged NgR level in the AD brain compared with controls. Two additional control and two additional AD cases had staining patterns similar to that shown here. Anti-actin immunoblot demonstrates similar protein loading. f, Sections of cerebral cortex or hippocampus from wild-type or APPswe/PSEN transgenic mice of 9 months age were stained with anti-NgR or nonimmune IgG (green in merged image) and anti-Aβ antibody (6E10, red in merged image). Brains were fixed in paraformaldehyde, sectioned in paraffin, and treated with formic acid before staining. In wild-type brain, axonal profiles are detected in the cerebral cortex, and cell soma are stained in the hippocampus (arrowheads). In transgenic brain, cellular and axonal staining is reduced, but periplaque NgR immunoreactivity (arrows) is detected.
Figure 2.
Physical interaction of NgR and APP. a, Epitope-tagged version of human APP and NgR were expressed in HEK-293T cells alone or in combination. Immunoprecipitates of APP show detectable NgR, and immunoprecipitates of NgR exhibit APP immunoreactivity. Twelve percent of the input protein is loaded in the bottom panels. b, Transfected COS-7 cells expressing Flag-APP (red) and NgR (green) were examined by double-labeled immunohistochemistry. c, APP and NgR localization in neurons. Mouse postnatal day 5 DRG neurons were stained with anti-APP (green) and anti-NgR antibodies (red). The insets show higher magnification of one growth cone. d, Rat brain homogenates were immunoprecipitated with 1D9 (monoclonal anti-NgR), control IgG, 5352 (anti-C-terminal-APP), and 51–2700 (anti-C-terminal-APP). Coimmunoprecipitation of APP and NgR was detected by immunoblotting with the indicated antibodies. Seven percent of the input protein is loaded in the indicated lanes. e, Particulate fractions of adult rat brain were treated for 1 h at 4°C with 3 m
m
BS3. Detergent solubilized protein was immunoprecipitated with anti-NgR antibody as in**d**. The immunoprecipitates were examined for NgR and APP immunoreactivity. Note the formation of a high-molecular-weight complex that is immunoreactive for both NgR and APP.
Figure 3.
APP ectodomain binds to NgR1-expressing cells. a, A schematic illustrates the cellular orientation of APP and the fragments that were used to express AP fusion proteins. b, COS-7 cells were cultured with transfection of human NgR1 or human NgR3 expression plasmid, and their respective expressions were verified by epitope-tag immunohistology. Fifty nanomolar solutions of AP-APP607, AP-APP579, and Aβ28-AP were allowed to bind to transfected cells for 2 h before washing, fixation, heat inactivation of endogenous AP, and enzymatic detection of bound fusion protein. Note the dark reaction product by arrowhead derived in cultures expressing NgR1 but not NgR3. An untransfected cell is labeled by the arrow. This is one of five experiments with similar results. c,d, Binding of AP-Aβ28, APP579, and APP607 to NgR1-expressing COS-7 cells from an experiment as in**a** was measured as a function of ligand concentration from 5 to 1250 n
m
. Data are means ± SEM from one of three experiments with similar results. The calculated_K_d is noted in d.e, Purified NgR-ecto(310)-Fc, MAG-Fc, or BSA (100 ng of each protein) was coated onto microtiter wells. After blockade of nonspecific sites with excess BSA, the wells were exposed to 50 n
m
AP, AP-Nogo 33, or AP-APP607. Data are the means ± SEM from three similar experiments. Note the selective binding of either Nogo(1–33) or APP607 to NgR-coated wells.f, The binding of 100 n
m
biotin-Aβ1–40 or biotin-Aβ40–1 to immobilized NgR(344)ecto-Fc or IgG (100 ng of each protein) was detected by retention of streptavidin-AP.
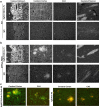
Figure 4.
Aβ1–40 binds to NgR-expressing cells.a–d, Binding of 100 n
m
biotinylated Aβ1–40 to COS-7 cells transfected with vector or rat NgR expression plasmid. Bound Aβ was detected by fluorescence-activated cell sorting (FACS) after incubation with Alexa488-conjugated streptavidin (a). Some cells were treated with 1 U of PI-PLC for 1 h at 37°C to release GPI-anchored proteins from the cell surface immediately before binding (a,c). The gating criterion for the quantitation of binding is illustrated by a bar, M. The dose dependence for binding was assessed with the indicated concentrations of Aβ (b). The NgR1 antibody 7E11 was added before biotinylated 100 n
m
Aβ to displace binding from NgR-expressing COS-7 cells (d).e–h, The fluorescence-activated cell sorting method was used to detect biotin-Aβ1–40 binding to human neuroblastoma SKNMC cells (e). Aβ1–40 bound dose dependently (f) and the binding was diminished after PI-PLC treatment (e, g).h, Biotinylated Aβ1–40 was displaced from SKNMC by anti-NgR antibodies (2E10, 1 μ
m
) and by soluble NgR(344)ecto-Fc (1 μ
m
). Data are means ± SEM from three to six experiments with similar results.
Figure 5.
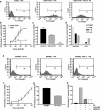
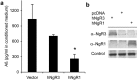
NgR expression decreases Aβ formation by neuroblastoma cells. Neuroblastoma N2A cells stably expressing APPswe (Thinakaran et al., 1996) were transiently transfected with control plasmid or vectors directing the expression of hNgR3 or hNgR1. a, Aβ1–40 ELISA was performed on conditioned medium. *p ≤ 0.025; the decrease in Aβ is significant and represents data from four independent transfections.b, N2A cell lysate was examined by immunoblot with the indicated antibodies to verify expression.
Figure 6.
Enhanced Aβ and neuritic dystrophy in FAD transgenic mice lacking NgR. Mice coexpressing human APPswe/PSEN-1(ΔE9) and heterozygous for a null allele of NgR were intercrossed with NgR−/− mice. At 6 months of age, littermate-matched mice expressing APPswe/PSEN-1(ΔE9) and either NgR+/− or_NgR−/−_ were killed. Brain tissue was analyzed by immunohistochemistry and ELISA. a,b, Arrows indicate examples of plaque deposits in hippocampal dentate gyrus and cerebral cortex. Plaque densities were quantified from nine mice in each group and are significantly different (*p_≤ 0.05, Student’s t test).c, Aβ1–40 and Aβ1–42 levels were assessed by ELISA and are significantly different (*p ≤ 0.04 level, Student’s_t test) for nine mice of each genotype.d, e, Anti-synaptophysin-immunoreactive dystrophic neurites are illustrated (arrows,d). The percentage of area occupied by synaptophysin-positive dystrophic neurites is reported in**e**. Data are means ± SEM from nine mice per group. *p < 0.05, Student’s t test; the decrease in dystrophic neurites is significant.
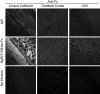
Figure 7.
NgR(310)ecto-Fc binding sites in myelinated tracts and Aβ plaque in brain sections. a, The binding of 0.1 μg/μl NgR(310)ecto-Fc or rat IgG to adult mouse brain sections was detected by fluorescent anti-rat IgG. Note the specific staining of myelinated tracts, prominent in corpus callosum and caudate–putamen. Intracortical and hippocampal fibers are labeled to a lesser degree. Brains were fixed in paraformaldehyde, sectioned in paraffin, and treated with formic acid before staining.b, In brain sections from APPsw/PSEN-1 double-transgenic mice of 9 months of age, the pattern of myelinated tract staining by NgR(310)ecto-Fc but not IgG is similar to that in the wild-type (WT) animals of**a. At this concentration, NgR(310)ecto-Fc, and to a lesser extent IgG, also binds to plaque-like structures.c**, Double immunohistochemistry of rat NgR(310)ecto-Fc or rat IgG (green) with anti-Aβ (6E10, red). Both rat NgR(310)ecto-Fc and rat IgG bind to dense Aβ-positive plaque (yellow), but NgR(310)ecto-Fc is also enriched at zones of lower Aβ at the edges of plaques (adjacent green).
Figure 8.
Localization of NgR(310)ecto-Fc after intracerebroventricular infusion. Brain sections were prepared from APPsw/PSEN-1 double-transgenic mice of 7 months of age after infusion with rat IgG, rat NgR(310)ecto-Fc, or no protein. The distribution of the infused protein was detected with fluorescently tagged anti-rat Fc and is similar to that of in vitro protein binding in Figure 6.
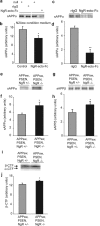
Figure 9.
NgR-ecto-Fc decreases Aβ plaque formation and dystrophic neurites in vivo. Transgenic mice expressing both the human APPswe and PSEN-1(ΔE9) protein were treated from 6–8 months of age with either rat IgG or rat NgR(310)ecto-Fc from a minipump connected to an intracerebroventricular catheter. Each mouse received 0.4 mg of protein over 2 months (0.29 mg · kg−1 · d−1).a, b, Transgenic mice were processed for Aβ immunohistology to reveal amyloid plaque deposition at 8 months of age (a). The arrows indicate examples of plaque deposits in sections of the cerebral cortex from three representative mice of eight or seven mice in each group. The area of cerebral cortex occupied by plaque was quantitated (b). Data are means ± SEM from_n_ = 8 or 7 mice. The decrease in Aβ-positive plaques in the NgR(310)ecto-Fc group compared with control is significant (**_p_≤ 0.02, Student’s t test).c, Aβ was measured in formic acid extracts of brain. Data are means ± SEM from nine animals in each group. The decrease in Aβ in the NgR(310)ecto-Fc group compared with IgG is significant (**p ≤ 0.02, Student’s t test). The ratio of Aβ40 to Aβ42 is not altered in the NgR(310)ecto-Fc-treated group. d, Correlation of plaque density and Aβ40 or Aβ42 levels in mice treated as in a–c.e, f, Anti-synaptophysin-immunoreactive dystrophic neurites in control and NgR(310)ecto-Fc-treated transgenic hippocampus are illustrated (arrows,e). The percentage of area occupied by synaptophysin-positive dystrophic neurites is reported in**f**. Data are means ± SEM from eight or seven mice per group. **p < 0.02, Student’s _t_test; the decrease in dystrophic neurites with NgR(310)ecto-Fc treatment is significant.
Figure 10.
NgR regulates sAPPα in parallel with Aβ levels. The level of sAPPα in brain extracts or N2A culture medium was analyzed by immunoprecipitation with anti-N-terminal-APP 22C11 antibody and immunoblot with anti-Aβ1–17 6E10 antibody. The sAPPβ fragment was analyzed in brain tissue by direct immunoblots with anti-sAPPβswe C-terminal antibody, and the CTFs were detected by the anti-APP-C-terminal 369 antibody. Immunoreactivity per milligram of protein was assessed in independent samples for_n_ = 6–9 mice or cell cultures in each experimental group (mean ± SEM; *p < 0.05 level, **p < 0.01, Student’s t test). a,b, sAPPα levels in conditioned medium from APPswe-expressing N2A cells was quantitated after 48 h culture in the presence of 1 mg/ml control rat IgG or rat NgR(310)ecto-Fc. c,d, sAPPα in brain extracts from APPswe/PSEN-1(ΔE9) transgenic mice treated from 6–8 months of age with either rat IgG or rat NgR(310)ecto-Fc intracerebroventricularly, as in Figure 9. e,f, sAPPα in brain extracts from 6-month-old littermate-matched mice transgenic for APPswe/PSEN-1(ΔE9) and either_NgR+/−_ or_NgR−/−_, as in Figure 6. g,h, sAPPβ in brain extracts from 13-month-old littermate-matched mice transgenic for APPswe/PSEN-1(ΔE9) and either_NgR+/−_ or_NgR−/−_.i, j, α-CTF and β-CTF in brain extracts from 13-month-old littermate-matched mice transgenic for APPswe/PSEN-1(ΔE9) and either NgR+/− or_NgR−/−_. The β-CTF level is quantitated in j.
Similar articles
- Nogo receptor interacts with brain APP and Abeta to reduce pathologic changes in Alzheimer's transgenic mice.
Park JH, Strittmatter SM. Park JH, et al. Curr Alzheimer Res. 2007 Dec;4(5):568-70. doi: 10.2174/156720507783018235. Curr Alzheimer Res. 2007. PMID: 18220524 Free PMC article. Review. - Subcutaneous Nogo receptor removes brain amyloid-beta and improves spatial memory in Alzheimer's transgenic mice.
Park JH, Widi GA, Gimbel DA, Harel NY, Lee DH, Strittmatter SM. Park JH, et al. J Neurosci. 2006 Dec 20;26(51):13279-86. doi: 10.1523/JNEUROSCI.4504-06.2006. J Neurosci. 2006. PMID: 17182778 Free PMC article. - The blockage of the Nogo/NgR signal pathway in microglia alleviates the formation of Aβ plaques and tau phosphorylation in APP/PS1 transgenic mice.
Fang Y, Yao L, Li C, Wang J, Wang J, Chen S, Zhou XF, Liao H. Fang Y, et al. J Neuroinflammation. 2016 Mar 3;13(1):56. doi: 10.1186/s12974-016-0522-x. J Neuroinflammation. 2016. PMID: 26939570 Free PMC article. - Interaction between amyloid precursor protein and Nogo receptors regulates amyloid deposition.
Zhou X, Hu X, He W, Tang X, Shi Q, Zhang Z, Yan R. Zhou X, et al. FASEB J. 2011 Sep;25(9):3146-56. doi: 10.1096/fj.11-184325. Epub 2011 Jun 13. FASEB J. 2011. PMID: 21670066 Free PMC article. - Nogo and Nogo receptor: relevance to schizophrenia?
Willi R, Schwab ME. Willi R, et al. Neurobiol Dis. 2013 Jun;54:150-7. doi: 10.1016/j.nbd.2013.01.011. Epub 2013 Jan 28. Neurobiol Dis. 2013. PMID: 23369871 Review.
Cited by
- The Nogo receptor 2 is a novel substrate of Fbs1.
Kern F, Sarg B, Stasyk T, Hess D, Lindner H. Kern F, et al. Biochem Biophys Res Commun. 2012 Jan 20;417(3):977-81. doi: 10.1016/j.bbrc.2011.12.050. Epub 2011 Dec 20. Biochem Biophys Res Commun. 2012. PMID: 22206664 Free PMC article. - Huntingtin and Other Neurodegeneration-Associated Proteins in the Development of Intracellular Pathologies: Potential Target Search for Therapeutic Intervention.
Churkina Taran AS, Shakhov AS, Kotlobay AA, Alieva IB. Churkina Taran AS, et al. Int J Mol Sci. 2022 Dec 8;23(24):15533. doi: 10.3390/ijms232415533. Int J Mol Sci. 2022. PMID: 36555175 Free PMC article. Review. - The amyloid precursor protein: beyond amyloid.
Zheng H, Koo EH. Zheng H, et al. Mol Neurodegener. 2006 Jul 3;1:5. doi: 10.1186/1750-1326-1-5. Mol Neurodegener. 2006. PMID: 16930452 Free PMC article. - Nogo receptor interacts with brain APP and Abeta to reduce pathologic changes in Alzheimer's transgenic mice.
Park JH, Strittmatter SM. Park JH, et al. Curr Alzheimer Res. 2007 Dec;4(5):568-70. doi: 10.2174/156720507783018235. Curr Alzheimer Res. 2007. PMID: 18220524 Free PMC article. Review. - Functional outcome is impaired following traumatic brain injury in aging Nogo-A/B-deficient mice.
Marklund N, Morales D, Clausen F, Hånell A, Kiwanuka O, Pitkänen A, Gimbel DA, Philipson O, Lannfelt L, Hillered L, Strittmatter SM, McIntosh TK. Marklund N, et al. Neuroscience. 2009 Oct 6;163(2):540-51. doi: 10.1016/j.neuroscience.2009.06.042. Epub 2009 Jun 23. Neuroscience. 2009. PMID: 19555742 Free PMC article.
References
- Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS (1997). Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron 19:939–945. - PubMed
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME (2000). Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403:434–439. - PubMed
- Fournier AE, GrandPre T, Strittmatter SM (2001). Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 409:341–346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 NS033020/NS/NINDS NIH HHS/United States
- R01 NS056485/NS/NINDS NIH HHS/United States
- R01 NS039962-10/NS/NINDS NIH HHS/United States
- R01 NS042304/NS/NINDS NIH HHS/United States
- R37 NS033020-17/NS/NINDS NIH HHS/United States
- R01 NS056485-04/NS/NINDS NIH HHS/United States
- R01 NS039962/NS/NINDS NIH HHS/United States
- R01 NS042304-08/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous