The chimeric Arabidopsis CYCLIC NUCLEOTIDE-GATED ION CHANNEL11/12 activates multiple pathogen resistance responses - PubMed (original) (raw)
The chimeric Arabidopsis CYCLIC NUCLEOTIDE-GATED ION CHANNEL11/12 activates multiple pathogen resistance responses
Keiko Yoshioka et al. Plant Cell. 2006 Mar.
Abstract
To investigate the resistance signaling pathways activated by pathogen infection, we previously identified the Arabidopsis thaliana mutant constitutive expresser of PR genes22 (cpr22), which displays constitutive activation of multiple defense responses. Here, we identify the cpr22 mutation as a 3-kb deletion that fuses two cyclic nucleotide-gated ion channel (ATCNGC)-encoding genes, ATCNGC11 and ATCNGC12, to generate a novel chimeric gene, ATCNGC11/12. Genetic, molecular, and complementation analyses suggest that ATCNGC11/12, as well as ATCNGC11 and ATCNGC12, form functional cAMP-activated ATCNGCs and that the phenotype conferred by cpr22 is attributable to the expression of ATCNGC11/12. However, because overexpression of ATCNGC12, but not ATCNGC11, suppressed the phenotype conferred by cpr22, the development of this phenotype appears to be regulated by the ratio between ATCNGC11/12 and ATCNGC12. Analysis of knockout lines revealed that both ATCNGC11 and ATCNGC12 are positive mediators of resistance against an avirulent biotype of Hyaloperonospora parasitica. Through epistatic analyses, cpr22-mediated enhanced resistance to pathogens was found to require NDR1-dependent and EDS1/PAD4-dependent pathways. In striking contrast, none of these pathways was required for cpr22-induced salicylic acid accumulation or PR-1 gene expression. These results demonstrate that NDR1, EDS1, and PAD4 mediate other resistance signaling function(s) in addition to salicylic acid and pathogenesis-related protein accumulation. Moreover, the requirement for both NDR1-dependent and EDS1/PAD4-dependent pathways for cpr22-mediated resistance suggests that these pathways are cross-regulated.
Figures
Figure 1.
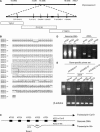
cpr22 Is a 3-kb Deletion That Creates a Chimeric ATCNGC-Encoding Gene. (A) Genetic and physical map of the cpr22 locus on chromosome 2. The top line represents chromosome 2; the bottom line is a larger scale map of the _cpr22_-containing region, with various markers indicated below the line and the number of recombination events for each marker indicated above it. The location of the 3-kb deletion is indicated as a black box. BAC clones T3F17, F11C10, and F13A10, which span the _cpr22_-containing region, are represented as open boxes below the map. (B) Amino acid sequence alignment of ATCNGC11, ATCNGC11/12, and ATCNGC12 from the Ws ecotype. The deduced amino acid sequence for ATCNGC11/12 is in the middle; amino acid residues in ATCNGC11 and/or ATCNGC12 that are identical to those of ATCNGC11/12 are represented as asterisks, whereas residues that differ are indicated above or below the line. The boxed region contains sequences that are identical in all three proteins. Because the predicted ATCNGC11/12 sequence is identical to ATCNGC11 upstream of the boxed sequence and to ATCNGC12 downstream of this sequence, the homologous recombination event that created ATCNGC11/12 likely occurred within this region. (C) Scheme of the 5′ translated and untranslated regions of the Col-0 and Ws alleles of ATCNGC11. To investigate the position of the translational start codon in the Ws allele of ATCNGC11, RT-PCR analysis was performed with primers corresponding to five ATG codons near the predicted translational start codon of the Col-0 allele. ATG1 to ATG5 are depicted as black boxes on the genomic DNA. This analysis suggested that the transcript for the Ws allele (bottom arrow) contains an extra 98-nucleotide intron and 27-nucleotide exon that are not present in the transcript of the Col-0 allele (top arrow). (D) PCR analysis of ATCNGC11 (Ws ecotype). Forward primers ATG1, ATG2, and ATG3, which correspond to the first three ATG codons within the predicted 5′ untranslated region of ATCNGC11, were tested for their ability to generate a product using genomic DNA or cDNA from wild-type Ws plants as the template in combination with the ATCNGC11-specific reverse primer UD1R1. ATG1 is closest to the annotated translational start codon, and ATG3 is farther upstream. The products were resolved on an agarose gel and stained with ethidium bromide. See text for additional information. (E) Semiquantitative RT-PCR analysis of ATCNGC11, ATCNGC12, and ATCNGC11/12 transcript levels in wild-type Ws and cpr22 homozygous plants using gene-specific primers. The products were resolved on an agarose gel and stained with ethidium bromide. β-Tubulin was used as a loading control.
Figure 2.
KO Lines of ATCNGC11 (Salk_026568), ATCNGC12 (Salk_ 092657 and Salk_092622), and Their F1 Progeny Display No Phenotypes Conferred by cpr22 but Exhibit Reduced Resistance to H. parasitica Emwa1. (A) Morphological phenotype and spontaneous cell death in wild-type Col-0 and Ws plants, the cpr22 mutant, ATCNGC11 and ATCNGC12 KO lines, and their F1 progeny. Plants were photographed 3 weeks after planting for single KO lines and 4 weeks after planting for F1 progeny. Lesion formation was monitored microscopically in leaves of 25-d-old plants after trypan blue staining. (B) RT-PCR analysis for PR-1 gene expression. PR1-F and PR1-R primers were used for the detection of PR-1 gene expression, and β-tubulin-5′ and β-tubulin-3′ primers were used for the detection of β-tubulin gene expression. (C) Infection with H. parasitica Emwa1. Seven-day-old seedlings were inoculated with H. parasitica Emwa1 (106 spores/mL). At 6 d after inoculation, two cotyledons per plant were analyzed with a microscope and categorized into one of five categories (0, 1 to 4, 5 to 10, 11 to 20, or >20) depending on the number of sporangiophores observed on the two cotyledons. Experiments were performed four times with similar results.
Figure 3.
Expression of ATCNGC11/12 Is Required for the Phenotype Conferred by cpr22. (A) Mutant morphology and spontaneous lesion formation in wild-type Ws plants transformed with ATCNGC11/12 driven by the CaMV 35S promoter. Plants were grown on soil and photographed at 4 weeks after planting . Microscopic analysis of trypan blue–stained leaves from these plants revealed intensely stained areas of dead cells. (B) Transient expression of ATCNGC12, ATCNGC11/12, or ATCNGC11 in N. benthamiana. Agrobacteria containing an empty vector (pMBP3) or a vector containing ATCNGC12, ATCNGC11, or ATCNGC11/12 driven by the CaMV 35S promoter were infiltrated into N. benthamiana leaves. The infiltrated areas are circled. Leaves were photographed at 3 d after infiltration and showed lesion formation only with ATCNGC11/12 expression. (C) Phenotypes of wild-type triploid (CPR22/CPR22/CPR22) and a triploid cpr22/CPR22/CPR22 plant. The plants were grown on soil and photographed at 3 weeks after planting (top). RNA gel blot analysis was performed using 8 μg of total RNA harvested from 3-week-old plants (bottom). Ethidium bromide staining of rRNA served as a loading control.
Figure 4.
The Severity of the Phenotype Conferred by cpr22 Is Determined by the Ratio between ATCNGC11/12 and ATCNGC12. (A) Morphological phenotypes of wild-type (CPR22/CPR22) and cpr22 (cpr22/CPR22) plants, as well as those of a cpr22 heterozygous plant transformed with an ∼5.5-kb genomic copy of ATCNGC11 (cpr22/CPR22 + ATCNGC11) and cpr22 heterozygous and homozygous plants transformed with an ∼3.3-kb genomic copy of ATCNGC12 (cpr22/CPR22 + ATCNGC12 and cpr22/cpr22 + ATCNGC12, respectively). The plants were grown on soil and photographed at 4 weeks after planting. (B) PR-1 gene expression in wild-type (CPR22/CPR22) plants, the cpr22/CPR22 mutant, and a cpr22/CPR22 mutant transformed with ATCNGC12. RNA gel blot analysis was performed using 8 μg of total RNA harvested from 3-week-old plants. Ethidium bromide staining of rRNA served as a loading control. (C) Transient coexpression analysis of ATCNGC11, ATCNGC12, or ATCNGC11/12 in N. benthamiana. Agrobacteria containing a 35S-ATCNGC11/12 construct in the pMBP3 vector were infiltrated alone or coinfiltrated with differing concentrations of agrobacteria containing either an empty vector or a 35S-ATCNGC12 or 35S-ATCNGC11 construct into N. benthamiana leaves. The ratios of ATCNGC11/12 to empty vector, ATCNGC12, and ATCNGC11 are designated at right. The infiltrated leaf was photographed at 3 d after infiltration. The expression level of each gene was confirmed and monitored by protein gel blot analysis using a specific antibody against green fluorescent protein and by fluorescent microscopic observation.
Figure 5.
ATCNGC11, ATCNGC12, and ATCNGC11/12 Encode Functional Cyclic Nucleotide-Gated Cation Channels. Complementation of hygromycin hypersensitivity of trk1,2 yeast by transfection with empty plasmid, ATCNGC11, ATCNGC12, or ATCNGC11/12. After transformation, the ability of yeast cells to grow around a filter disk containing 3 M KCl (center of each panel) on solid YPGal medium containing 0.07 mg/mL hygromycin B in the presence or absence of 100 μM cAMP was monitored. Photographs were taken after 3 d of growth. Data shown are representative results obtained with this assay in four independent experiments.
Figure 6.
The cpr22 Mutant Develops a Normal HR after Infection with an Avirulent Pathogen. Microscopic analysis of trypan blue–stained leaves from ∼20-d-old wild-type Ws and cpr22/CPR22 plants. After inoculation with P. syringae pv tomato carrying avrRpt2, leaves were photographed at 0, 2, or 5 d after inoculation (DAI).
Figure 7.
PAD4, EDS1, and NDR1 Are Not Required for _cpr22_-Mediated Stunting, Spontaneous Lesion Formation, or PR-1 Gene Expression. (A) Comparison of the morphology displayed by segregating F3 progeny from a self-pollinated cpr22/CPR22 mutant plant and those of self-pollinated cpr22/CPR22 plants homozygous for ndr1-1, pad4-1, or eds1-1 (cpr22 ndr1-1, cpr22 pad4-1, and cpr22 eds1-1, respectively). Arrows indicate plants containing the cpr22/CPR22 genotype (based on molecular and progeny analyses); regardless of the allele at the NDR1, PAD4, or EDS1 locus, these plants displayed stunted growth and curly leaves. Plants were photographed at 4 weeks after planting. (B) Microscopic analysis of trypan blue–stained leaves from 3-week-old F3 progeny heterozygous for cpr22 as well as from cpr22/CPR22 plants homozygous for ndr1-1, pad4-1, or eds1-1. Darkly stained areas indicative of cell death were detected in all sets of progeny. (C) Expression of the PR-1 gene in CPR22/CPR22 (Ws-wt and Col-wt) plants, heterozygous cpr22/CPR22 (cpr22) and homozygous ndr1-1, pad4-1, or eds1-1 single mutant plants, and cpr22 heterozygous and ndr1-1, pad4-1, or eds1-1 homozygous double mutants. RNA gel blot analysis was performed using 8 μg of total RNA harvested from 3-week-old soil-grown plants. Ethidium bromide staining of rRNA was used as a loading control.
Figure 8.
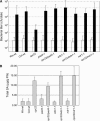
EDS1, PAD4, and NDR1 Are Required for _cpr22_-Mediated Resistance to P. syringae pv maculicola ES4326 but Not for SA Accumulation. (A) After infiltration with P. syringae pv maculicola ES4326, bacterial growth was monitored in the leaves of 3-week-old wild-type Ws and Col-0 plants, cpr22/CPR22, eds1-1, pad4-1, and ndr1-1 single mutants, and cpr22 heterozygous, ndr1-1, pad4-1, or eds1-1 homozygous double mutant plants by collecting three leaf discs at 0 d after inoculation (open bars) and 3 d after inoculation (closed bars). Colony-forming units (cfu) are expressed ±
sd
and represent averages of four independent samples. (B) Total SA levels were assayed in leaves of 3-week-old, soil-grown wild-type Ws and Col-0 plants, cpr22/CPR22 (c_pr22_), eds1-1, pad4-1, and ndr1-1 single mutants, and cpr22 heterozygous, ndr1-1, pad4-1, or eds1-1 homozygous double mutant plants. The values are presented in micrograms per gram fresh weight (FW) and represent averages ±
sd
of three samples consisting of leaves from three plants per line.
Similar articles
- Identification of a functionally essential amino acid for Arabidopsis cyclic nucleotide gated ion channels using the chimeric AtCNGC11/12 gene.
Baxter J, Moeder W, Urquhart W, Shahinas D, Chin K, Christendat D, Kang HG, Angelova M, Kato N, Yoshioka K. Baxter J, et al. Plant J. 2008 Nov;56(3):457-69. doi: 10.1111/j.1365-313X.2008.03619.x. Epub 2008 Aug 14. Plant J. 2008. PMID: 18643993 - The chimeric cyclic nucleotide-gated ion channel ATCNGC11/12 constitutively induces programmed cell death in a Ca2+ dependent manner.
Urquhart W, Gunawardena AH, Moeder W, Ali R, Berkowitz GA, Yoshioka K. Urquhart W, et al. Plant Mol Biol. 2007 Dec;65(6):747-61. doi: 10.1007/s11103-007-9239-7. Epub 2007 Sep 21. Plant Mol Biol. 2007. PMID: 17885810 - A suppressor screen of the chimeric AtCNGC11/12 reveals residues important for intersubunit interactions of cyclic nucleotide-gated ion channels.
Abdel-Hamid H, Chin K, Moeder W, Shahinas D, Gupta D, Yoshioka K. Abdel-Hamid H, et al. Plant Physiol. 2013 Jul;162(3):1681-93. doi: 10.1104/pp.113.217539. Epub 2013 Jun 4. Plant Physiol. 2013. PMID: 23735507 Free PMC article. - The role of NDR1 in pathogen perception and plant defense signaling.
Knepper C, Savory EA, Day B. Knepper C, et al. Plant Signal Behav. 2011 Aug;6(8):1114-6. doi: 10.4161/psb.6.8.15843. Epub 2011 Aug 1. Plant Signal Behav. 2011. PMID: 21758001 Free PMC article. Review. - Genetic dissection of R gene signal transduction pathways.
Innes RW. Innes RW. Curr Opin Plant Biol. 1998 Aug;1(4):299-304. doi: 10.1016/1369-5266(88)80050-5. Curr Opin Plant Biol. 1998. PMID: 10066602 Review.
Cited by
- Comparative Transcriptome Analysis between Resistant and Susceptible Pakchoi Cultivars in Response to Downy Mildew.
Chen Y, Miao L, Li X, Liu Y, Xi D, Zhang D, Gao L, Zhu Y, Dai S, Zhu H. Chen Y, et al. Int J Mol Sci. 2023 Oct 28;24(21):15710. doi: 10.3390/ijms242115710. Int J Mol Sci. 2023. PMID: 37958694 Free PMC article. - High throughput chemical screening supports the involvement of Ca2+ in cyclic nucleotide-gated ion channel-mediated programmed cell death in Arabidopsis.
Abdel-Hamid H, Chin K, Moeder W, Yoshioka K. Abdel-Hamid H, et al. Plant Signal Behav. 2011 Nov;6(11):1817-9. doi: 10.4161/psb.6.11.17502. Epub 2011 Nov 1. Plant Signal Behav. 2011. PMID: 22041991 Free PMC article. - CYCLIC NUCLEOTIDE-GATED ION CHANNELs 14 and 16 Promote Tolerance to Heat and Chilling in Rice.
Cui Y, Lu S, Li Z, Cheng J, Hu P, Zhu T, Wang X, Jin M, Wang X, Li L, Huang S, Zou B, Hua J. Cui Y, et al. Plant Physiol. 2020 Aug;183(4):1794-1808. doi: 10.1104/pp.20.00591. Epub 2020 Jun 11. Plant Physiol. 2020. PMID: 32527735 Free PMC article. - Chickpea transcription factor CaTLP1 interacts with protein kinases, modulates ROS accumulation and promotes ABA-mediated stomatal closure.
Wardhan V, Pandey A, Chakraborty S, Chakraborty N. Wardhan V, et al. Sci Rep. 2016 Dec 9;6:38121. doi: 10.1038/srep38121. Sci Rep. 2016. PMID: 27934866 Free PMC article. - Phylogeny and evolution of plant cyclic nucleotide-gated ion channel (CNGC) gene family and functional analyses of tomato CNGCs.
Saand MA, Xu YP, Munyampundu JP, Li W, Zhang XR, Cai XZ. Saand MA, et al. DNA Res. 2015 Dec;22(6):471-83. doi: 10.1093/dnares/dsv029. Epub 2015 Nov 5. DNA Res. 2015. PMID: 26546226 Free PMC article.
References
- Arazi, T., Sunkar, R., Kaplan, B., and Fromm, H. (1999). A tobacco plasma membrane calmodulin-binding transporter confers Ni2+ tolerance and Pb2+ hypersensitivity in transgenic plants. Plant J. 20 171–182. - PubMed
- Balague, C., Lin, B., Alcon, C., Flottes, G., Malmstrom, S., Kohler, C., Neuhaus, G., Pelletier, G., Gaymard, F., and Roby, D. (2003). HLM1, an essential signaling component in the hypersensitive response, is a member of the cyclic nucleotide-gated channel ion channel family. Plant Cell 15 365–379. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials