S-nitrosothiol depletion in amyotrophic lateral sclerosis - PubMed (original) (raw)
. 2006 Feb 14;103(7):2404-9.
doi: 10.1073/pnas.0507243103. Epub 2006 Feb 6.
Masaaki Matsuoka, Hemachand Tummala, Michael A Johnson, Alvaro G Estevéz, Rui Wu, Andrés Kamaid, Karina C Ricart, Yuichi Hashimoto, Benjamin Gaston, Timothy L Macdonald, Zuoshang Xu, Joan B Mannick
Affiliations
- PMID: 16461917
- PMCID: PMC1413693
- DOI: 10.1073/pnas.0507243103
S-nitrosothiol depletion in amyotrophic lateral sclerosis
Christopher M Schonhoff et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Recent data suggest that either excessive or deficient levels of protein S-nitrosylation may contribute to disease. Disruption of S-nitrosothiol (SNO) homeostasis may result not only from altered nitric oxide (NO) synthase activity but also from alterations in the activity of denitrosylases that remove NO groups. A subset of patients with familial amyotrophic lateral sclerosis (ALS) have mutations in superoxide dismutase 1 (SOD1) that increase the denitrosylase activity of SOD1. Here, we show that the increased denitrosylase activity of SOD1 mutants leads to an aberrant decrease in intracellular protein and peptide S-nitrosylation in cell and animal models of ALS. Deficient S-nitrosylation is particularly prominent in the mitochondria of cells expressing SOD1 mutants. Our results suggest that SNO depletion disrupts the function and/or subcellular localization of proteins that are regulated by S-nitrosylation such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and thereby contributes to ALS pathogenesis. Repletion of intracellular SNO levels with SNO donor compounds rescues cells from mutant SOD1-induced death. These results suggest that aberrant depletion of intracellular SNOs contributes to motor neuron death in ALS, and raises the possibility that deficient S-nitrosylation is a general mechanism of disease pathogenesis. SNO donor compounds may provide new therapeutic options for diseases such as ALS that are associated with deficient S-nitrosylation.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
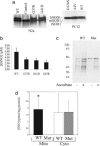
Fig. 1.
Decreased peptide and protein S-nitrosylation in cell lines expressing SOD1 mutants. (a) SOD1 expression levels in N2a or PC12 cells stably transfected with empty control vector (Control), or vectors expressing human WT SOD1 (WT), or ALS-associated SOD1 mutants (G37R, G41D, D124N, or A4V). Bands corresponding to human SOD1 (hSOD1) and endogenous mouse (mSOD1) or rat SOD1 (rSOD1) are shown. (b) GSNO levels in cell lysates obtained from N2a cells expressing either WT or mutant (G37R, G41D, G85R) SOD1 were measured by LC-MS. The data represent the mean ± SEM of four to six independent experiments. The pooled GSNO values in cells expressing SOD1 mutants are significantly lower than the GSNO values in cells expressing WT SOD1 (P = 0.01, unpaired two-tailed t test). (c) Whole-cell lysates from PC12 cells expressing WT or D124N mutant (Mut) SOD1 were treated with 1 μM GSNO for 1 h at room temperature in the dark. Protein S-nitrosylation in the lysates was then determined by using a biotin switch assay. Biotin labeling that increases in the presence of ascorbate (+ascorbate) is indicative of protein S-nitrosylation. The gel is representative of three comparable experiments. (d) Total SNOs in mitochondrial (Mito) and cytoplasmic (Cyto) lysates of N2a cells expressing WT or mutant (Mut) SOD1 (G37R and G41D) were measured by chemiluminescence. ∗, P = 0.03 versus mutant mitochondria, paired t test, n = 8.
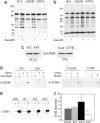
Fig. 2.
Total protein and GAPDH S-nitrosylation are decreased in cell lines and the spinal cords of transgenic mice expressing SOD1 mutants. (a) Total protein S-nitrosylation in spinal cord lysates obtained from G85R and G93A mutant SOD1 transgenic mice at the time of paralysis or from age-matched WT SOD1 transgenic mice was assessed by the biotin switch method. Increased biotin labeling after ascorbate treatment of samples (+ascorbate) is indicative of protein S-nitrosylation. The data are representative of six separate experiments. (b) The experiment described in a was repeated using mitochondrial lysates obtained from the spinal cords of G85R and G93A mice pre-disease onset and from age-matched WT transgenic control mice. The data are representative of two separate experiments. (c) Levels of GAPDH in whole-cell lysates of PC12 cells expressing WT or A4V mutant SOD1, or N2a cells expressing control vector (Cont) or G37R mutant SOD1 were determined by GAPDH immunoblot analysis. The data are representative of three to four separate experiments for each cell line. (d) Lysates of PC12 cells expressing WT or A4V mutant SOD1, or N2a cells expressing control vector (Control) or G37R mutant SOD1 were treated with (+) or without (−) 40 μM GSNO for 1 h in the dark at room temperature. Protein S-nitrosylation was then assessed by using the biotin switch method. As controls, ascorbate or biotin were not added to some samples. The data are representative of three (N2a cells) or four (PC12 cells) separate experiments. (e) GAPDH S-nitrosylation was assessed by the biotin switch assay in spinal cords obtained from mutant (G85R or G93A) SOD1 transgenic mice at the onset of muscle weakness or from age-matched WT SOD1 transgenic (WT) or nontransgenic (NTG) control mice. Biotin labeling in the absence (−) and presence (+) of ascorbate and total levels of GAPDH in the starting lysates (S) of each sample are shown. The data are representative of four separate experiments. (f) Relative levels of GAPDH S-nitrosylation as obtained by densitometric analysis of biotin switch assays described in e. The data represent the mean ± SEM of ascorbate-induced increased biotin labeling of GAPDH over background biotin labeling from three to five separate experiments. ∗, P = 0.016 versus WT, 2-tailed t test for independent samples.
Fig. 3.
Translocation of GAPDH into the nucleus and mitochondria is decreased in cells and transgenic mice expressing SOD1 mutants. (a) Levels of GAPDH in equal concentrations of cytoplasmic (Cyto), nuclear (Nucleus), or mitochondrial (Mito) lysates of PC12 cells expressing WT or A4V mutant SOD1 were assessed on GAPDH immunoblots. As loading controls and to assess the purity of the subcellular fractions, lamin (nucleus), 14-13-3Β (cytoplasm) and cytochrome c (cyto c, mitochondria) immunoblots of the subcellular fractions were performed. The experiment is representative of three (mitochondria) or eight (nucleus) separate experiments. Similar results were obtained by using PC12 cells expressing D124N mutant SOD1. (b) Immunoblots of GAPDH levels in equal concentrations of nuclear (Nucleus) or mitochondrial (Mito) fractions obtained from the spinal cords of G93A (G93) or G85R (G85) mice pre-disease onset or from age-matched WT transgenic controls (WT) are shown. Lysate indicates unfractionated whole-cell WT lysate. The data are representative of two separate experiments. (c) PC12 cells expressing A4V SOD1 were exposed to SNOC for 0, 15, or 30 min. Levels of GAPDH in the nuclear and cytoplasmic fraction were then determined by GAPDH immunoblot analysis. The data are representative of five separate experiments.
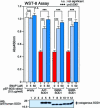
Fig. 4.
Repletion of intracellular SNO levels with SNO donor compounds protects against mutant SOD1-induced toxicity. NSC34 cells were transiently transfected with empty vector (empty) or vectors expressing the SOD1 mutants A4T, G85R, or G93R. After transfection, the cells were treated with 0, 5, or 50 μM SNAP. After 72 h, cell viability was determined by the WST-8 assay, and mutant SOD1 expression was determined on immunoblots. The numbered lanes on the immunoblots correspond to samples 1–12 in the WST-8 assay. The data represent the mean ± SD, n = 3. Statistical analysis was performed with one-way factorial ANOVA followed by Scheffé’s probable least-squares difference (PLSD). ∗∗∗, P < 0.001.
Similar articles
- S-nitrosylated protein disulfide isomerase contributes to mutant SOD1 aggregates in amyotrophic lateral sclerosis.
Chen X, Zhang X, Li C, Guan T, Shang H, Cui L, Li XM, Kong J. Chen X, et al. J Neurochem. 2013 Jan;124(1):45-58. doi: 10.1111/jnc.12046. Epub 2012 Nov 1. J Neurochem. 2013. PMID: 23043510 - Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1.
Jaarsma D, Haasdijk ED, Grashorn JA, Hawkins R, van Duijn W, Verspaget HW, London J, Holstege JC. Jaarsma D, et al. Neurobiol Dis. 2000 Dec;7(6 Pt B):623-43. doi: 10.1006/nbdi.2000.0299. Neurobiol Dis. 2000. PMID: 11114261 - Histological evidence of protein aggregation in mutant SOD1 transgenic mice and in amyotrophic lateral sclerosis neural tissues.
Watanabe M, Dykes-Hoberg M, Culotta VC, Price DL, Wong PC, Rothstein JD. Watanabe M, et al. Neurobiol Dis. 2001 Dec;8(6):933-41. doi: 10.1006/nbdi.2001.0443. Neurobiol Dis. 2001. PMID: 11741389 - Misfolded SOD1 and ALS: zeroing in on mitochondria.
Pickles S, Vande Velde C. Pickles S, et al. Amyotroph Lateral Scler. 2012 Jun;13(4):333-40. doi: 10.3109/17482968.2012.648645. Epub 2012 Apr 3. Amyotroph Lateral Scler. 2012. PMID: 22471903 Review. - Mechanisms of neurodegeneration in amyotrophic lateral sclerosis.
Cluskey S, Ramsden DB. Cluskey S, et al. Mol Pathol. 2001 Dec;54(6):386-92. Mol Pathol. 2001. PMID: 11724913 Free PMC article. Review.
Cited by
- The inhibitory effect of S-nitrosoglutathione on blood-brain barrier disruption and peroxynitrite formation in a rat model of experimental stroke.
Khan M, Dhammu TS, Sakakima H, Shunmugavel A, Gilg AG, Singh AK, Singh I. Khan M, et al. J Neurochem. 2012 Nov;123 Suppl 2(Suppl 2):86-97. doi: 10.1111/j.1471-4159.2012.07947.x. J Neurochem. 2012. PMID: 23050646 Free PMC article. - Abnormal SDS-PAGE migration of cytosolic proteins can identify domains and mechanisms that control surfactant binding.
Shi Y, Mowery RA, Ashley J, Hentz M, Ramirez AJ, Bilgicer B, Slunt-Brown H, Borchelt DR, Shaw BF. Shi Y, et al. Protein Sci. 2012 Aug;21(8):1197-209. doi: 10.1002/pro.2107. Protein Sci. 2012. PMID: 22692797 Free PMC article. - Commentary: mechanistic considerations for associations between formaldehyde exposure and nasopharyngeal carcinoma.
Thompson CM, Grafström RC. Thompson CM, et al. Environ Health. 2009 Nov 25;8:53. doi: 10.1186/1476-069X-8-53. Environ Health. 2009. PMID: 19939253 Free PMC article. - iSNO-PseAAC: predict cysteine S-nitrosylation sites in proteins by incorporating position specific amino acid propensity into pseudo amino acid composition.
Xu Y, Ding J, Wu LY, Chou KC. Xu Y, et al. PLoS One. 2013;8(2):e55844. doi: 10.1371/journal.pone.0055844. Epub 2013 Feb 7. PLoS One. 2013. PMID: 23409062 Free PMC article. - S-nitrosylation of critical protein thiols mediates protein misfolding and mitochondrial dysfunction in neurodegenerative diseases.
Nakamura T, Lipton SA. Nakamura T, et al. Antioxid Redox Signal. 2011 Apr 15;14(8):1479-92. doi: 10.1089/ars.2010.3570. Epub 2011 Jan 8. Antioxid Redox Signal. 2011. PMID: 20812868 Free PMC article. Review.
References
- Chung K. K., Thomas B., Li X., Pletnikova O., Troncoso J. C., Marsh L., Dawson V. L., Dawson T. M. Science. 2004;304:1328–1331. - PubMed
- Gu Z., Kaul M., Yan B., Kridel S. J., Cui J., Strongin A., Smith J. W., Liddington R. C., Lipton S. A. Science. 2002;297:1186–1190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS36761/NS/NINDS NIH HHS/United States
- R01 NS042834/NS/NINDS NIH HHS/United States
- NS41739/NS/NINDS NIH HHS/United States
- R01 NS041739/NS/NINDS NIH HHS/United States
- R01 NS036761/NS/NINDS NIH HHS/United States
- R21 NS049212/NS/NINDS NIH HHS/United States
- NS42834/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous