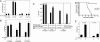Prion protein is expressed on long-term repopulating hematopoietic stem cells and is important for their self-renewal - PubMed (original) (raw)
Prion protein is expressed on long-term repopulating hematopoietic stem cells and is important for their self-renewal
Cheng Cheng Zhang et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Although the wild-type prion protein (PrP) is abundant and widely expressed in various types of tissues and cells, its physiological function(s) remain unknown, and PrP knockout mice do not exhibit overt and undisputed phenotypes. Here we showed that PrP is expressed on the surface of several bone marrow cell populations successively enriched in long-term (LT) hematopoietic stem cells (HSCs) using flow cytometry analysis. Affinity purification of the PrP-positive and -negative fractions from these populations, followed by competitive bone marrow reconstitution assays, shows that all LT HSCs express PrP. HSCs from PrP-null bone marrow exhibited impaired self-renewal in serial transplantation of lethally irradiated mouse recipients both in the presence and absence of competitors. When treated with a cell cycle-specific myelotoxic agent, the animals reconstituted with PrP-null HSCs exhibit increased sensitivity to hematopoietic cell depletion. Ectopic expression of PrP in PrP-null bone marrow cells by retroviral infection rescued the defective hematopoietic engraftment during serial transplantation. Therefore, PrP is a marker for HSCs and supports their self-renewal.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
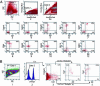
PrP is expressed on bone marrow populations enriched in HSC activity. (A) Freshly isolated BM cells were stained with Hoechst dye 33342, and the SP fraction was gated (gates 1 and 2) to analyze the expression of PrP. In plot 1, forward scatter (FSC) and side scatter (SSC) is used to gate on hematopoietic cells. Hoechst Red and Hoechst Blue (plots 2 and 2a, gate 2 was set as 0.02% of total cells) represent two fluorescence emission wavelengths used to detect the SP cells. Plot 2a is an expansion of the gate 2 region of plot 2. PrP-null BM SP cells served as a negative control for PrP antibody staining (plot 3). WT BM SP cells were stained for PrP (plots 4–12) together with isotype control (plot 4), Endoglin (plot 5), Sca-1 (plot 6), CD43 (plot 7), CD44 (plot 8), CD49D (plot 9), CD49E (plot 10), CD11A (plot 11), or CD62L (plot 12). (B) Total BM cells were stained with anti-Endoglin followed sequentially by anti-rat-PE/CY5.5, a mixture of biotinylated lineage-specific antibodies, and streptavidin–APC, anti-PrP-FITC, and anti-Sca-1-PE. Plot 1 shows the gate of FSC and SSC channels. The lowest 5% of APC-stained cells (i.e., Lin−) were gated (plot 2). Plot 3 shows the staining of the gated Lin− cells with Sca-1 and Endoglin, and plot 4 shows the staining of the gated Lin− Sca-1+ Endoglin+ cells with PrP. Plot 5 shows the staining of the gated Lin− Sca-1+ Endoglin− cells with PrP.
Fig. 2.
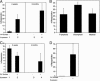
All LT repopulating bone marrow HSC cells express surface PrP. A total of 1 × 105 PrP− or 2 × 104 PrP+ CD45.2 donor BM cells (A), 2 × 104 PrP+ CD45.2 donor BM cells (B), 500 sorted Lin−Sca-1+PrP− or 100 Lin−Sca-1+PrP+ CD45.2 donor BM cells (C), or 500 isolated SP PrP− or 250 SP PrP+ donor BM cells (D) were mixed with 1 × 105 competitor CD45.1 cells and transplanted into lethally irradiated CD45.1 mice (n = 4–5). (A) Donor CD45.2 contribution at 4 weeks and 6 months after transplant. (B) Multilineage contribution at 6 months after transplant. (C) Donor contribution at 3 weeks and 4 months after transplant. (D) Donor contribution at 4 months after transplant.
Fig. 3.
Ectopic expression of PrP rescues the impaired self-renewal of PrP-null stem cells during serial transplantation. (A) Colony assays for erythroid, lymphoid, and myeloid progenitors in PrP-null and WT BM. Total BM cell populations were plated in methylcellulose medium M3434 (StemCell Technologies) for quantifying CFU-GEMM, CFU-GM, and BFU-E colonies, and in M3630 (StemCell Technologies) for quantifying CFU-Pre-B colonies. (B) Serial transplantation of PrP-null and WT BM with competitor bone marrow cells. A total of 2 × 106 donor CD45.2 PrP-null or littermate control BM cells were mixed with 2 × 106 CD45.1 WT BM cells and transplanted into lethally irradiated CD45.1 recipients (n = 6). The extent of chimerism in peripheral blood (bars 1 and 2) was analyzed 4 months after transplant. In the experiment in bars 3 and 4, BM cells from the primary transplanted mice were pooled, and 2 × 106 cells were injected directly into each of five lethally irradiated CD45.1 recipients. The fraction of donor CD45.2 cells in the peripheral blood of these transplanted mice was analyzed 4 months later. The process was repeated for the tertiary transplants (bars 5 and 6). This is a combined result of three independent experiments from a total of initial six null or wild-type control mice. ∗, Significantly different from bar 3 value, P < 0.005; ∗∗, significantly different from bar 5 value, _P_ < 0.005. (_C_) Serial transplantation of PrP-null and WT BM cells without competitors; rescue of HSC activity in PrP-null cells by PrP expression. A total of 1 × 106 PrP-null or WT BM CD45.2 cells, pooled from three donors, were transplanted into lethally irradiated CD45.1 recipients without competitors. Recipients were monitored daily for survival for >30 days (bars 1 and 2, n = 6). These mice were killed after 4 months. From them, 5 × 105 BM cells were collected and transplanted into new irradiated recipients (bars 3 and 4, n = 7). The process was repeated an additional time for tertiary transplants (bars 5 and 6, n = 12). In parallel, 1 × 106 PrP-null BM cells isolated from the surviving secondary transplant recipients, as shown in bar 3, were infected by retroviruses encoding GFP, PrP, or PrP Δ23-72, and injected into irradiated recipients (bars 7–9, n = 7–8). Plotted is the fraction of surviving mice 50 days after each bone marrow transplant. See Fig. 6 for details of animal survival. (D) A total of 1 × 106 BM cells from the secondary transplanted mice shown in bars 3 and 4 of C were transplanted into the lethally irradiated recipients. Survival data were plotted as Kaplan–Meier curves (n = 11 for each group, P < 0.0001, log-rank test). (E) Competitive transplantation demonstrates impaired renewal of PrP-null HSC activity during successive bone marrow transplants. Here, 5 × 105 PrP-null or WT BM collected from primary transplanted mice 4 months after transplant (without competitors, as in C, bars 1 and 2) were mixed with 5 × 105 CD45.1 freshly isolated BM cells and transplanted into lethally irradiated recipients. Peripheral blood engraftment at 6 weeks and 5 months after transplant is shown (n = 4). ∗, Significantly different from bar 1 value, P < 0.005; ∗∗, significantly different from bar 3 value, P < 0.05.
Fig. 4.
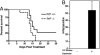
PrP-null HSCs are more sensitive than normal to myelotoxic injury. (A) A total of 5 × 105 CD45.2 PrP-null or wild-type BM cells, pooled from three littermates, were transplanted into lethally irradiated CD45.1 recipients. One month after transplant, 11 recipient mice from each group were treated with 150 mg/kg 5-FU i.p. weekly for 2 weeks. The survival of these two groups was analyzed by using a log-rank nonparametric test (P = 0.0307, n = 11 in each group) and expressed as Kaplan–Meier survival plot. (B) 5-FU (150 mg/kg) was administered i.p. to three wild-type CD45.2 mice. After 3 days, the BM of these treated mice was pooled and fractionated according to PrP expression. A total of 1 × 105 PrP+ or 1 × 106 PrP× BM cells were transplanted, together with 2 × 105 competitor CD45.1 wild-type BM cells, into lethally irradiated CD45.1 recipients. The level of chimerism in the peripheral blood of the recipients was analyzed 4 months later (n = 5).
Similar articles
- A novel function of interleukin-10 promoting self-renewal of hematopoietic stem cells.
Kang YJ, Yang SJ, Park G, Cho B, Min CK, Kim TY, Lee JS, Oh IH. Kang YJ, et al. Stem Cells. 2007 Jul;25(7):1814-22. doi: 10.1634/stemcells.2007-0002. Epub 2007 Apr 26. Stem Cells. 2007. PMID: 17464085 - Quantification of self-renewal capacity in single hematopoietic stem cells from normal and Lnk-deficient mice.
Ema H, Sudo K, Seita J, Matsubara A, Morita Y, Osawa M, Takatsu K, Takaki S, Nakauchi H. Ema H, et al. Dev Cell. 2005 Jun;8(6):907-14. doi: 10.1016/j.devcel.2005.03.019. Dev Cell. 2005. PMID: 15935779 - Dormant and self-renewing hematopoietic stem cells and their niches.
Wilson A, Oser GM, Jaworski M, Blanco-Bose WE, Laurenti E, Adolphe C, Essers MA, Macdonald HR, Trumpp A. Wilson A, et al. Ann N Y Acad Sci. 2007 Jun;1106:64-75. doi: 10.1196/annals.1392.021. Epub 2007 Apr 18. Ann N Y Acad Sci. 2007. PMID: 17442778 Review. - Shp-2 heterozygous hematopoietic stem cells have deficient repopulating ability due to diminished self-renewal.
Chan RJ, Li Y, Hass MN, Walter A, Voorhorst CS, Shelley WC, Yang Z, Orschell CM, Yoder MC. Chan RJ, et al. Exp Hematol. 2006 Sep;34(9):1230-9. doi: 10.1016/j.exphem.2006.04.017. Exp Hematol. 2006. PMID: 16939816 - Physiological role of the cellular prion protein.
Zomosa-Signoret V, Arnaud JD, Fontes P, Alvarez-Martinez MT, Liautard JP. Zomosa-Signoret V, et al. Vet Res. 2008 Jul-Aug;39(4):9. doi: 10.1051/vetres:2007048. Epub 2007 Nov 27. Vet Res. 2008. PMID: 18073096 Review.
Cited by
- Histamine stimulates human microglia to alter cellular prion protein expression via the HRH2 histamine receptor.
Pehar M, Hewitt M, Wagner A, Sandhu JK, Khalili A, Wang X, Cho JY, Sim VL, Kulka M. Pehar M, et al. Sci Rep. 2024 Oct 26;14(1):25519. doi: 10.1038/s41598-024-75982-1. Sci Rep. 2024. PMID: 39462031 Free PMC article. - Prion protein alters viral control and enhances pathology after perinatal cytomegalovirus infection.
Karner D, Kvestak D, Kucan Brlic P, Cokaric Brdovcak M, Lisnic B, Brizic I, Juranic Lisnic V, Golemac M, Tomac J, Krmpotic A, Karkeni E, Libri V, Mella S, Legname G, Altmeppen HC, Hasan M, Jonjic S, Lenac Rovis T. Karner D, et al. Nat Commun. 2024 Sep 5;15(1):7754. doi: 10.1038/s41467-024-51931-4. Nat Commun. 2024. PMID: 39237588 Free PMC article. - Prion protein E219K polymorphism: from the discovery of the KANNO blood group to interventions for human prion disease.
Wang SS, Meng ZL, Zhang YW, Yan YS, Li LB. Wang SS, et al. Front Neurol. 2024 Jul 10;15:1392984. doi: 10.3389/fneur.2024.1392984. eCollection 2024. Front Neurol. 2024. PMID: 39050130 Free PMC article. Review. - Potential Therapeutic Use of Stem Cells for Prion Diseases.
Zayed M, Kook SH, Jeong BH. Zayed M, et al. Cells. 2023 Oct 7;12(19):2413. doi: 10.3390/cells12192413. Cells. 2023. PMID: 37830627 Free PMC article. Review. - Prion infection modulates hematopoietic stem/progenitor cell fate through cell-autonomous and non-autonomous mechanisms.
Sim HJ, Kim YC, Bhattarai G, Won SY, Lee JC, Jeong BH, Kook SH. Sim HJ, et al. Leukemia. 2023 Apr;37(4):877-887. doi: 10.1038/s41375-023-01828-w. Epub 2023 Jan 27. Leukemia. 2023. PMID: 36707620 Free PMC article.
References
- Jordan C. T., McKearn J. P., Lemischka I. R. Cell. 1990;61:953–963. - PubMed
- Rebel V. I., Miller C. L., Thornbury G. R., Dragowska W. H., Eaves C. J., Lansdorp P. M. Exp. Hematol. 1996;24:638–648. - PubMed
- Spangrude G. J., Heimfeld S., Weissman I. L. Science. 1988;241:58–62. - PubMed
- Chen C. Z., Li L., Li M., Lodish H. F. Immunity. 2003;19:525–533. - PubMed
- Zhang C. C., Lodish H. F. Blood. 2004;103:2513–2521. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials