Activated radixin is essential for GABAA receptor alpha5 subunit anchoring at the actin cytoskeleton - PubMed (original) (raw)
Activated radixin is essential for GABAA receptor alpha5 subunit anchoring at the actin cytoskeleton
Sven Loebrich et al. EMBO J. 2006.
Abstract
Neurotransmitter receptor clustering is thought to represent a critical parameter for neuronal transmission. Little is known about the mechanisms that anchor and concentrate inhibitory neurotransmitter receptors in neurons. GABAA receptor (GABAAR) alpha5 subunits mainly locate at extrasynaptic sites and are thought to mediate tonic inhibition. Notably, similar as synaptic GABAARs, these receptor subtypes also appear in cluster formations at neuronal surface membranes and are of particular interest in cognitive processing. GABAAR alpha5 mutation or depletion facilitates trace fear conditioning or improves spatial learning in mice, respectively. Here, we identified the actin-binding protein radixin, a member of the ERM family, as the first directly interacting molecule that anchors GABAARs at cytoskeletal elements. Intramolecular activation of radixin is a functional prerequisite for GABAAR alpha5 subunit binding and both depletion of radixin expression as well as replacement of the radixin F-actin binding motif interferes with GABAAR alpha5 cluster formation. Our data suggest radixin to represent a critical factor in receptor localization and/or downstream signaling.
Figures
Figure 1
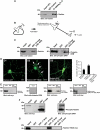
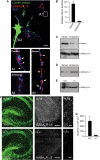
Identification of radixin as an interactor of GABAAR α5 subunits in the yeast two-hybrid screen. (A) Mapping of the radixin binding site on the large intracellular loop of GABAAR α5 (bait). (B) Radixin domain structure. The fragment isolated from the yeast two-hybrid screen is located in the FERM domain (black bar). (C) Alignment of the amino-acid sequence of rat radixin (NM_001005889) with the sequence encoded by the positive clone from the screen. (D) Intracellular TMIII-TMIV loops of the GABAA receptor subunits α1, α3, β2 and γ2 were tested for radixin interaction.
Figure 2
Intramolecular activation of radixin is required for GABAAR α5 subunit binding. (A) Co-immunoprecipitation of radixin and GABAAR α5 from rat brain extract. (B) Schematic representation of radixin intramolecular activation upon phosphorylation of threonine 564. Arrows: sites of mutagenesis; K: lysine; T: threonine. (C) Pulldown assays from rat brain extract with immobilized GABAAR α5 loop fused to GST. (D) Neuronal localization of wild-type and mutant radixin proteins and quantification of clusters per 30 μm dendrite. Arrows depict prominent membrane localization. (E) GST-pulldown assays with myc-tagged radixin wild-type and mutant proteins upon heterologous expression in HEK293 cells. (F) Combinatorial application of kinase activators and the phosphatase inhibitor Calyculin A leads to radixin activation in COS cells. The form of radixin, which binds to the GABAAR α5 loop is phosphorylated at threonine 564, as detected with a phospho ERM-specific antibody. (G) Intracellular TMIII-TMIV loops of the GABAAR subunits α1, α3, β2 and γ2 are not able to bind the active radixin mutant in GST-pulldown assays. Scale bars: 20 μm.
Figure 3
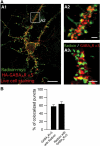
Colocalization of radixin (green) and GABAAR α5 (red) in neuronal dendrites. (A) Coimmunostaining of cultured hippocampal neurons expressing endogenous (A3) or tagged versions of radixin and GABAAR α5 (A1 and A2). As shown in A1 and A2, prior to fixation, neurons were incubated with anti-HA antibody to ensure surface staining of the receptor. (B) Quantification of endogenous GABAAR α5 subunit colocalization with endogenous radixin and vice versa. Scale bars: 20 μm (A1); 3 μm (A2, A3).
Figure 4
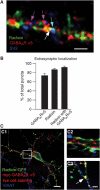
Synaptic and extrasynaptic localization of radixin, GABAAR α5 and radixin/GABAAR α5 coclusters. (A) Triple immunostaining of endogenous radixin (green), endogenous GABAAR α5 (red) and the synaptic marker SV2 (blue) on mature cultured hippocampal neurons. Colored arrows depict examples of colocalization. (B) Quantification of extrasynaptic radixin, GABAAR α5 and radixin/ GABAAR α5 coclusters. (C) Neuronal expression of radixin-GFP and myc-tagged GABAAR α5 (red). Prior to fixation, neurons were incubated with anti-myc antibody to ensure surface detection of the receptor. Radixin/GABAAR α5 coclusters are occasionally found at VIAAT-positive inhibitory synapses (white, arrow). Scale bars: 15 μm (C1); 4 μm (A, C3).
Figure 5
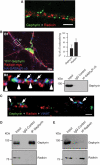
Radixin and gephyrin represent independent systems. (A) Immunostaining of endogenous radixin (red) and endogenous gephyrin (green) on a dendritic region. (B) Triple expression of YFP-gephyrin (green), radixin-myc (red) and HA-GABAAR α5 (blue) in cultured hippocampal neurons. Note that GABAAR α5 subunits colocalize either with radixin (arrowheads) or with gephyrin (arrows). Quantification of HA-GABAAR α5 colocalization with radixin-myc and YFP-gephyrin (upper right). Endogenous gephyrin does not co-immunoprecipitate with endogenous GABAAR α5 under our experimental conditions (lower right). (C) Triple immunostaining of endogenous gephyrin, radixin and VIAAT. Only about 14% of radixin clusters are found at VIAAT-positive inhibitory synapses. Arrows: gephyrin-positive synapses. (D) Immunoprecipitation of gephyrin from rat brain lysate. (E) Immunoprecipitation of radixin from rat brain lysate. Scale bars: 2 μm (A), 12 μm (B1) 1 μm (C).
Figure 6
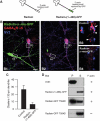
Knockdown or depletion of radixin expression leads to loss of GABAAR α5 clustering. (A) Top: An antisense oligonucleotide injected (green) and a noninjected cell are compared. Synapses are represented by SV2 (blue). The immunoreactivity for GABAAR α5 is diffuse in antisense-treated cells (A2), as compared to normal receptor clusters in untreated (A1) or sense oligonucleotide-treated cells (A3). Arrowheads and arrows depict extrasynaptic and synaptic clusters, respectively. (B) Quantification of GABAAR α5 cluster density in sense- and antisense-injected cells. (C) Whole-cell extracts from cultured hippocampal neurons confirm that radixin expression is efficiently downregulated upon antisense treatment, while total GABAAR α5 expression is unaltered. (D) Western Blot analysis of biotinylated surface GABAAR α5 in sense- or antisense-transfected neurons. (E) Western Blot analysis of P2 plasma membrane preparations from wild-type or radixin knockout mice. (F) Immunohistochemical analysis of wild-type and radixin knockout hippocampal slices. Left: TOTO staining to visualize overall tissue structure. Middle and right: GABAAR α5 staining reveals loss of GABAAR α5 receptor clusters with remaining diffuse signals upon radixin deficiency. (G) Quantification of GABAAR α5 subunit clusters between radixin +/+ and −/− genotypes (P<0.05). Scale bars: 10 μm in (A), 200 μm in (F) (middle) and 10 μm in (F) (right).
Figure 7
Deletion of the F-actin-binding site in radixin leads to loss of GABAAR α5 clustering. (A) Schematic drawing of the truncated molecule. The F-actin-binding motif is replaced by GFP. (B) Hippocampal neurons expressing Radixin-(1–468)-GFP. A transfected (green) and a nontransfected control cell are shown (B1). B2: Same image without the green channel. Green arrow: transfected cell; arrow: synaptic; arrowheads: extrasynaptic. Scale bar (B): 20 μm. (C) Quantification of GABAAR α5 cluster density in control and transfected cells. (D) Characterization of F-actin-binding capacity of different radixin constructs in a cosedimentation assay. Plus and minus indicates the presence or absence of F-actin in the experiment. P: pellet, S: supernatant.
Figure 8
Tonic and quantal synaptic GABAergic currents in the presence of the F-actin binding-deficient mutant Radixin-(1–468)-GFP. Tonic GABAergic currents were determined by the application of 100 μM bicuculline methiodite (Bic) as the difference in holding current (dashed lines in A and B). Owing to cell variabilities, representative experiments are shown for two GFP-expressing (A) and two Radixin-(1–468)-GFP-expressing neurons (B) with small (upper traces) and large tonic current components (lower traces). (C) Summary of tonic current measurements derived from GFP-expressing (_n_=24) and Radixin-(1–468)-GFP (_n_=31)-expressing neurons. Horizontal lines represent mean values (GFP: 145 pA; Radixin-(1–468)-GFP: 176 pA). (D) Representative GABAergic miniature inhibitory postsynaptic currents (mIPSCs) recorded from a GFP- and a Radixin-(1–468)-GFP-expressing neuron. (E) Averaged mIPSCs from representative GFP (left trace, 379 events averaged)- and Radixin-(1–468)-GFP (right trace, 368 events averaged)-expressing neurons (lines represent single-exponential fits). Bar graphs show mean 10–90% rise times (F), sizes (G) and decay time constants (H) for mIPSCs from GFP (_n_=17)- and Radixin-(1–468)-GFP-expressing neurons (_n_=18).
Similar articles
- Selective inhibition of extra-synaptic α5-GABAA receptors by S44819, a new therapeutic agent.
Etherington LA, Mihalik B, Pálvölgyi A, Ling I, Pallagi K, Kertész S, Varga P, Gunn BG, Brown AR, Livesey MR, Monteiro O, Belelli D, Barkóczy J, Spedding M, Gacsályi I, Antoni FA, Lambert JJ. Etherington LA, et al. Neuropharmacology. 2017 Oct;125:353-364. doi: 10.1016/j.neuropharm.2017.08.012. Epub 2017 Aug 12. Neuropharmacology. 2017. PMID: 28807671 - Radixin regulates synaptic GABAA receptor density and is essential for reversal learning and short-term memory.
Hausrat TJ, Muhia M, Gerrow K, Thomas P, Hirdes W, Tsukita S, Heisler FF, Herich L, Dubroqua S, Breiden P, Feldon J, Schwarz JR, Yee BK, Smart TG, Triller A, Kneussel M. Hausrat TJ, et al. Nat Commun. 2015 Apr 20;6:6872. doi: 10.1038/ncomms7872. Nat Commun. 2015. PMID: 25891999 Free PMC article. - The alpha5(H105R) mutation impairs alpha5 selective binding properties by altered positioning of the alpha5 subunit in GABAA receptors containing two distinct types of alpha subunits.
Balic E, Rudolph U, Fritschy JM, Mohler H, Benke D. Balic E, et al. J Neurochem. 2009 Jul;110(1):244-54. doi: 10.1111/j.1471-4159.2009.06119.x. Epub 2009 Apr 27. J Neurochem. 2009. PMID: 19457072 - GABAA receptor associated proteins: a key factor regulating GABAA receptor function.
Chen ZW, Olsen RW. Chen ZW, et al. J Neurochem. 2007 Jan;100(2):279-94. doi: 10.1111/j.1471-4159.2006.04206.x. Epub 2006 Nov 2. J Neurochem. 2007. PMID: 17083446 Review. - Ezrin/radixin/moesin: versatile controllers of signaling molecules and of the cortical cytoskeleton.
Niggli V, Rossy J. Niggli V, et al. Int J Biochem Cell Biol. 2008;40(3):344-9. doi: 10.1016/j.biocel.2007.02.012. Epub 2007 Feb 22. Int J Biochem Cell Biol. 2008. PMID: 17419089 Review.
Cited by
- Monoallelic loss of the F-actin-binding protein radixin facilitates startle reactivity and pre-pulse inhibition in mice.
Hausrat TJ, Vogl C, Neef J, Schweizer M, Yee BK, Strenzke N, Kneussel M. Hausrat TJ, et al. Front Cell Dev Biol. 2022 Nov 28;10:987691. doi: 10.3389/fcell.2022.987691. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36518539 Free PMC article. - Gephyrin: a master regulator of neuronal function?
Tyagarajan SK, Fritschy JM. Tyagarajan SK, et al. Nat Rev Neurosci. 2014 Mar;15(3):141-56. doi: 10.1038/nrn3670. Nat Rev Neurosci. 2014. PMID: 24552784 Review. - Altered inhibitory synapses in de novo GABRA5 and GABRA1 mutations associated with early onset epileptic encephalopathies.
Hernandez CC, XiangWei W, Hu N, Shen D, Shen W, Lagrange AH, Zhang Y, Dai L, Ding C, Sun Z, Hu J, Zhu H, Jiang Y, Macdonald RL. Hernandez CC, et al. Brain. 2019 Jul 1;142(7):1938-1954. doi: 10.1093/brain/awz123. Brain. 2019. PMID: 31056671 Free PMC article. - Alterations in GABAA-Receptor Trafficking and Synaptic Dysfunction in Brain Disorders.
Mele M, Costa RO, Duarte CB. Mele M, et al. Front Cell Neurosci. 2019 Mar 7;13:77. doi: 10.3389/fncel.2019.00077. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30899215 Free PMC article. - Comparative- and network-based proteomic analysis of bacterial chondronecrosis with osteomyelitis lesions in broiler's proximal tibiae identifies new molecular signatures of lameness.
Cook J, Greene ES, Ramser A, Mullenix G, Dridi JS, Liyanage R, Wideman R, Dridi S. Cook J, et al. Sci Rep. 2023 Apr 12;13(1):5947. doi: 10.1038/s41598-023-33060-y. Sci Rep. 2023. PMID: 37045932 Free PMC article.
References
- Albrecht J, Bender AS, Norenberg MD (1998) Potassium-stimulated GABA release is a chloride-dependent but sodium- and calcium-independent process in cultured astrocytes. Acta Neurobiol Exp (Wars) 58: 169–175 - PubMed
- Allenspach EJ, Cullinan P, Tong J, Tang Q, Tesciuba AG, Cannon JL, Takahashi SM, Morgan R, Burkhardt JK, Sperling AI (2001) ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity 15: 739–750 - PubMed
- Barakat L, Bordey A (2002) GAT-1 and reversible GABA transport in Bergmann glia in slices. J Neurophysiol 88: 1407–1419 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases