Activity-dependent regulation of synaptic strength and neuronal excitability in central auditory pathways - PubMed (original) (raw)
Review
Activity-dependent regulation of synaptic strength and neuronal excitability in central auditory pathways
Bruce Walmsley et al. J Physiol. 2006.
Abstract
Neural activity plays an important role in regulating synaptic strength and neuronal membrane properties. Attempts to establish guiding rules for activity-dependent neuronal changes have led to such concepts as homeostasis of cellular activity and Hebbian reinforcement of synaptic strength. However, it is clear that there are diverse effects resulting from activity changes, and that these changes depend on the experimental preparation, and the developmental stage of the neural circuits under study. In addition, most experimental evidence on activity-dependent regulation comes from reduced preparations such as neuronal cultures. This review highlights recent results from studies of the intact mammalian auditory system, where changes in activity have been shown to produce alterations in synaptic and membrane properties at the level of individual neurons, and changes in network properties, including the formation of tonotopic maps.
Figures
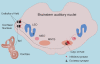
Figure 1. Major pathways in the mammalian auditory brainstem
This schematic shows the auditory nerve (AN) arising from the cochlea and making monosynaptic connections with neurons in the cochlea nucleus of the brainstem (AVCN – anteroventral cochlear nucleus). Also shown are some of the major brainstem auditory nuclei: the medial nucleus of the trapezoid body (MNTB), the medial superior olive (MSO) and the lateral superior olive (LSO). Auditory nerve fibres make large excitatory synaptic contacts, the endbulbs of Held, with bushy cells in the AVCN. Globular bushy cells in the AVCN, in turn, make large calyceal contacts, the calyces of Held, with principal cells in the contralateral MNTB. Spherical bushy cells make ipsilateral contacts with the dendrites of neurons in the MSO. Excitatory neurons and synapses are shown in black; inhibitory neurons and synapses are shown in red.
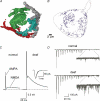
Figure 2. Excitatory synaptic transmission is greater in deaf mice
A, reconstructions from electron micrographs of 4 different endbulbs of Held contacting a bushy cell in the AVCN. B shows the individual synaptic specializations contained within the boutons shown in A. C shows that both AMPA and NMDA components of the synaptic current arising from individual auditory nerve fibres are larger in deaf (dn/dn) mice. D illustrates that delayed asynchronous spontaneous release is much larger in deaf mice (insets). (A adapted from Nicol & Walmsley, 2002, with permission from Blackwell Publishing Ltd, B–D adapted from Oleskevich & Walmsley, 2002, with permission from Blackwell Publishing Ltd.)
Figure 3. MNTB neurons are more excitable in MNTB neurons
A illustrates that MNTB neurons usually respond to depolarizing currents with a single or a few, action potentials, whereas MNTB neurons in deaf (dn/dn) mice respond with multiple action potentials. Panels on the left indicate the up- (arrows up), down- (arrows down) or no change (X) in the magnitude of voltage-activated currents in MNTB neurons from deaf cf. normal mice. Background immunolabelling shows HCN1 immunoreactivity (red) on LSO neurons (green).
Figure 4. Tonotopic gradients of channel expression are disrupted in deaf mice
A illustrates an obvious medial (M) to lateral (L) gradient in pre- and post-synaptic immunolabelling of Kv3.4 channels (red) in MNTB neurons from a normal mouse. B illustrates a summary schematic (adapted from Leao et al. 2005_a_, with permission from Blackwell Publishing Ltd) of the MNTB (green shading) in normal and deaf (dn/dn) mice. Left panel shows the tonotopic (HF, high frequency; LF, low frequency) gradient of high-threshold potassium currents (Kv3.1), low-threshold potassium currents (Kv1.1) and hyperpolarization-activated currents (HCN4). Right panel shows that these gradients are not present in deaf (dn/dn) mice.
Similar articles
- Hyperpolarization-activated (I) currents in auditory brainstem neurons of normal and congenitally deaf mice.
Leao RN, Svahn K, Berntson A, Walmsley B. Leao RN, et al. Eur J Neurosci. 2005 Jul;22(1):147-57. doi: 10.1111/j.1460-9568.2005.04185.x. Eur J Neurosci. 2005. PMID: 16029204 - Making brain circuits listen.
Rauschecker JP. Rauschecker JP. Science. 1999 Sep 10;285(5434):1686-7. doi: 10.1126/science.285.5434.1686. Science. 1999. PMID: 10523187 No abstract available. - The effects of long and short term profound deafness on the responses of inferior colliculus to electrical stimulation of the cochlea.
Shirane M, Harrison RV. Shirane M, et al. Acta Otolaryngol Suppl. 1991;489:32-40. doi: 10.3109/00016489109127705. Acta Otolaryngol Suppl. 1991. PMID: 1763644 - Afferent regulation of neurons in the brain stem auditory system.
Rubel EW, Hyson RL, Durham D. Rubel EW, et al. J Neurobiol. 1990 Jan;21(1):169-96. doi: 10.1002/neu.480210112. J Neurobiol. 1990. PMID: 2181062 Review. - Use it or lose it? Lessons learned from the developing brains of children who are deaf and use cochlear implants to hear.
Gordon KA, Wong DD, Valero J, Jewell SF, Yoo P, Papsin BC. Gordon KA, et al. Brain Topogr. 2011 Oct;24(3-4):204-19. doi: 10.1007/s10548-011-0181-2. Epub 2011 Apr 11. Brain Topogr. 2011. PMID: 21479928 Review.
Cited by
- Potential Therapeutic Effects of Bifidobacterium breve MCC1274 on Alzheimer's Disease Pathologies in AppNL-G-F Mice.
Abdelhamid M, Jung CG, Zhou C, Inoue R, Chen Y, Sento Y, Hida H, Michikawa M. Abdelhamid M, et al. Nutrients. 2024 Feb 15;16(4):538. doi: 10.3390/nu16040538. Nutrients. 2024. PMID: 38398861 Free PMC article. - Modulation of potassium conductances optimizes fidelity of auditory information.
Kaczmarek LK. Kaczmarek LK. Proc Natl Acad Sci U S A. 2023 Mar 21;120(12):e2216440120. doi: 10.1073/pnas.2216440120. Epub 2023 Mar 17. Proc Natl Acad Sci U S A. 2023. PMID: 36930599 Free PMC article. - Tooth Loss Induces Memory Impairment and Glial Activation in Young Wild-Type Mice.
Taslima F, Abdelhamid M, Zhou C, Chen Y, Jung CG, Michikawa M. Taslima F, et al. J Alzheimers Dis Rep. 2022 Nov 3;6(1):663-675. doi: 10.3233/ADR-220053. eCollection 2022. J Alzheimers Dis Rep. 2022. PMID: 36506484 Free PMC article. - Correlative Assembly of Subsynaptic Nanoscale Organizations During Development.
Sun SY, Li XW, Cao R, Zhao Y, Sheng N, Tang AH. Sun SY, et al. Front Synaptic Neurosci. 2022 May 24;14:748184. doi: 10.3389/fnsyn.2022.748184. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 35685244 Free PMC article. - Time Course of Activity-Dependent Changes in Auditory Nerve Synapses Reveals Multiple Underlying Cellular Mechanisms.
Wong NF, Xu-Friedman MA. Wong NF, et al. J Neurosci. 2022 Mar 23;42(12):2492-2502. doi: 10.1523/JNEUROSCI.1583-21.2022. Epub 2022 Feb 18. J Neurosci. 2022. PMID: 35181597 Free PMC article.
References
- Bacci A, Coco S, Pravettoni E, Schenk U, Armano S, Frassoni C, Verderio C, De Camilli P, Matteoli M. Chronic blockade of glutamate receptors enhances presynaptic release and downregulates the interaction between synaptophysin-synaptobrevin-vesicle-associated membrane protein 2. J Neurosci. 2001;21:6588–6596. - PMC - PubMed
- Barnes-Davies M, Barker MC, Osmani F, Forsythe ID. Kv1 currents mediate a gradient of principal neuron excitability across the tonotopic axis in the rat lateral superior olive. Eur J Neurosci. 2004;19:325–333. - PubMed
- Bock GR, Frank MP, Steel KP. Preservation of central auditory function in the deafness mouse. Brain Res. 1982;239:608–612. - PubMed
- Borst JG, Sakmann B. Calcium influx and transmitter release at a fast CNS synapse. Nature. 1996;383:431–434. - PubMed
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time difference coding. Nature. 2002;417:543–547. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources