Biosynthesis of plant volatiles: nature's diversity and ingenuity - PubMed (original) (raw)
Review
Biosynthesis of plant volatiles: nature's diversity and ingenuity
Eran Pichersky et al. Science. 2006.
Abstract
Plant volatiles (PVs) are lipophilic molecules with high vapor pressure that serve various ecological roles. The synthesis of PVs involves the removal of hydrophilic moieties and oxidation/hydroxylation, reduction, methylation, and acylation reactions. Some PV biosynthetic enzymes produce multiple products from a single substrate or act on multiple substrates. Genes for PV biosynthesis evolve by duplication of genes that direct other aspects of plant metabolism; these duplicated genes then diverge from each other over time. Changes in the preferred substrate or resultant product of PV enzymes may occur through minimal changes of critical residues. Convergent evolution is often responsible for the ability of distally related species to synthesize the same volatile.
Figures
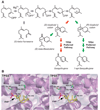
Fig. 1
Maize TPS4 and TPS5 catalyze the formation of the same complement of sesquiterpene products, albeit in distinctly different proportions. (A) Abbreviated TPS4 and TPS5 mechanisms accounting for the major products of each enzyme [29% (S)-β-bisabolene, 9% (E)-β-farnesene, 24% 7-epi-sequithujene, and 6% sesquithujene for TPS4; and 27% (S)-β-bisabolene, 13% (E)-β-farnesene, 2% 7-epi-sesquithujene, and 28% sesquithujene for TPS5]. For clarity, only the major products are shown or those products whose levels are considerably different for TPS4 and TPS5. OPP signifies a pyrophosphate. Gray arrows depict pathways equally shared among TPS4 and TPS5. Red and green arrows depict TPS5- and TPS4-preferred pathways, respectively [adapted from (12)]. (B) Active site models of TPS4 and TPS5 based on the crystal structure of tobacco 5-epi-aristolochene synthase (10), shown with the FPP nonhydrolyzable analog farnesyl hydroxyphosphonate (FHP). The four key residues responsible for functional divergence—Thr407, Ala409, Thr410, and Ile411 in TPS4, and Ser407, Gly409, Ala410, and Asn411 in TPS5— are shown as color-coded sticks, with the underlying secondary structure of the three-dimensional models colored lavender.
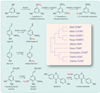
Fig. 2
Structural relatedness of some methyltransferases and their substrates. POMT, OOMT1, and OOMT2 are involved in synthesizing the rose floral volatile 1,3,5-trimethoxybenzene. Basil EOMT and C. breweri IEMT methylate eugenol to produce methyleugenol. Basil CVOMT methylates chavicol to produce methylchavicol. Eugenol, chavicol, methylchavicol, and methyleugenol are all volatiles with distinct aromas. COMT is an enzyme in the lignin biosynthetic pathway, and IOMT methylates the nonvolatile isoflavone daidzein to the nonvolatile isoformonentin. All these enzymes use _S_-adenosyl-
l
-methionine as the methyl donor [adapted from (–34)]. The branches in the schematic phylogenetic tree are not drawn to scale.
Fig. 3
Localization of storage and synthesis of PVs. (A) Conical cells on the surface of snapdragon petals synthesize and emit terpenes and benzenoids. (B) Glands of Cistus creticus, a shrub native to Crete, are rich in volatile and nonvolatile terpenes.
Fig. 4
Summary of the cellular processes involved in the synthesis of PVs. Most modification reactions occur in the cytosol, but some may take place in other subcellular compartments, including plastids, mitochondria, peroxisomes, and the endoplasmic reticulum.
Similar articles
- Plant volatiles: a lack of function or a lack of knowledge?
Pichersky E, Sharkey TD, Gershenzon J. Pichersky E, et al. Trends Plant Sci. 2006 Sep;11(9):421; author reply 422-3. doi: 10.1016/j.tplants.2006.07.007. Epub 2006 Aug 7. Trends Plant Sci. 2006. PMID: 16891148 No abstract available. - Multiple functions of inducible plant volatiles.
Holopainen JK. Holopainen JK. Trends Plant Sci. 2004 Nov;9(11):529-33. doi: 10.1016/j.tplants.2004.09.006. Trends Plant Sci. 2004. PMID: 15501177 Review. - Functional evolution of biosynthetic enzymes that produce plant volatiles.
Koeduka T. Koeduka T. Biosci Biotechnol Biochem. 2018 Feb;82(2):192-199. doi: 10.1080/09168451.2017.1422968. Epub 2018 Jan 17. Biosci Biotechnol Biochem. 2018. PMID: 29338642 Review. - Transcriptomic and metabolomic analyses provide insight into the volatile compounds of citrus leaves and flowers.
Zhang H, Chen M, Wen H, Wang Z, Chen J, Fang L, Zhang H, Xie Z, Jiang D, Cheng Y, Xu J. Zhang H, et al. BMC Plant Biol. 2020 Jan 6;20(1):7. doi: 10.1186/s12870-019-2222-z. BMC Plant Biol. 2020. PMID: 31906915 Free PMC article. - Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers.
Chen F, Tholl D, D'Auria JC, Farooq A, Pichersky E, Gershenzon J. Chen F, et al. Plant Cell. 2003 Feb;15(2):481-94. doi: 10.1105/tpc.007989. Plant Cell. 2003. PMID: 12566586 Free PMC article.
Cited by
- GC-MS Combined with Proteomic Analysis of Volatile Compounds and Formation Mechanisms in Green Teas with Different Aroma Types.
Niu X, Ao C, Yu J, Zhao Y, Huang H. Niu X, et al. Foods. 2024 Jun 13;13(12):1848. doi: 10.3390/foods13121848. Foods. 2024. PMID: 38928790 Free PMC article. - Volatile Organic Compounds Emitted by Flowers: Ecological Roles, Production by Plants, Extraction, and Identification.
Lo MM, Benfodda Z, Molinié R, Meffre P. Lo MM, et al. Plants (Basel). 2024 Jan 31;13(3):417. doi: 10.3390/plants13030417. Plants (Basel). 2024. PMID: 38337950 Free PMC article. Review. - Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature.
Heil M, Silva Bueno JC. Heil M, et al. Proc Natl Acad Sci U S A. 2007 Mar 27;104(13):5467-72. doi: 10.1073/pnas.0610266104. Epub 2007 Mar 7. Proc Natl Acad Sci U S A. 2007. PMID: 17360371 Free PMC article. - Natural variation meets synthetic biology: Promiscuous trichome-expressed acyltransferases from Nicotiana.
Schenck CA, Anthony TM, Jacobs M, Jones AD, Last RL. Schenck CA, et al. Plant Physiol. 2022 Aug 29;190(1):146-164. doi: 10.1093/plphys/kiac192. Plant Physiol. 2022. PMID: 35477794 Free PMC article. - A UDP-Glucose:Monoterpenol Glucosyltransferase Adds to the Chemical Diversity of the Grapevine Metabolome.
Bönisch F, Frotscher J, Stanitzek S, Rühl E, Wüst M, Bitz O, Schwab W. Bönisch F, et al. Plant Physiol. 2014 Jun;165(2):561-581. doi: 10.1104/pp.113.232470. Epub 2014 May 1. Plant Physiol. 2014. PMID: 24784757 Free PMC article.
References
- Pichersky E, Gershenzon J. Curr. Opin. Plant Biol. 2002;5:237. - PubMed
- Goodwin SM, et al. Physiol. Plant. 2003;117:435. - PubMed
- Pichersky E, Gang D. Trends Plant Sci. 2000;5:439. - PubMed
- Goff SA, Klee HJ. Science. 2006;311:XXXX. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials