Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference - PubMed (original) (raw)
Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference
Emmanuel Valjent et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Repeated association of drugs of abuse with context leads to long-lasting behavioral responses that reflect reward-controlled learning and participate in the establishment of addiction. Reactivation of consolidated memories is known to produce a reconsolidation process during which memories undergo a labile state. We investigated whether reexposure to drugs had similar effects. Cocaine administration activates extracellular signal-regulated kinase (ERK) in the striatum, and ERK activation is required for the acquisition of cocaine-induced conditioned place preference (CPP). When mice previously conditioned for cocaine-place preference were reexposed to cocaine in the drug-paired compartment after systemic administration of SL327, an inhibitor of ERK activation, CPP response was abolished 24 h later. This procedure also abolished the phosphorylation of ERK and glutamate receptor-1 observed in the ventral and dorsal striatum, 24 h later, during CPP test. Erasure of CPP by SL327 required the combination of cocaine administration and drug-paired context and did not result from enhanced extinction. Similarly, reexposure to morphine in the presence of SL327 long-lastingly abolished response of previously learned morphine-CPP. The effects of SL327 on cocaine- or morphine-CPP were reproduced by protein synthesis inhibition. In contrast, protein synthesis inhibition did not alter previously acquired locomotor sensitization to cocaine. Our findings show that an established CPP can be disrupted when reactivation associates both the conditioned context and drug administration. This process involves ERK, and systemic treatment preventing ERK activation during reexposure erases the previously learned behavioral response. These results suggest potential therapeutic strategies to explore in the context of addiction.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
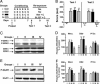
Reexposure to cocaine in drug-paired compartment in the presence of a MEK inhibitor erases CPP and its biochemical correlates. (A) Experimental design: S, saline; C, cocaine, 20 mg/kg; Veh, vehicle; SL327, 30 mg/kg. Each group included 8 mice. (B) After conditioning, mice developed a significant preference for the cocaine-paired side (test 1, F(3,28) = 16.77, P < 0.01). Inhibition of ERK activation by systemic administration of SL327 1 h before cocaine reexposure suppressed the place preference when animals were tested 24 h later (test 2, F(3,28) = 20.79, P < 0.01). Data are means ± SEM. Post hoc comparison (Bonferroni test), group I vs. group II to IV: ∗∗, P < 0.01; group III vs. group IV: ○○, P < 0.01. (C_–_E) To assess protein phosphorylation during the CPP test, P-ERK and total ERK immunoreactivity were analyzed at the end of test 2, by immunoblotting in NAcc (C), DStr, and prefrontal cortex (Pf Cx), and results were quantified (D). Phospho-Ser-845-GluR1 and total GluR1 were similarly analyzed (E and F). Results were expressed as a ratio of phosphoprotein/total protein and normalized as percentage of controls. Data are means ± SEM (seven to eight mice per group. One way ANOVA for P-ERK2 was as follows: NAcc, F(3,24) = 11.34, P < 0.01; DStr, F(3,27) = 9.41, P < 0.01; Pf Cx, F(3,26) = 4.98, P < 0.01. One way ANOVA for P-GluR1 was as follows: NAcc, F(3,25) = 10.46, P < 0.01; DStr, F(3,25) = 7.24, P < 0.01; Pf Cx, F(3,25) = 0.62, NS. Bonferroni test was as follows: group I vs. group II to IV, ∗, P < 0.05; ∗∗, P < 0.01; group III vs. group IV, ○○, P < 0.01).
Fig. 2.
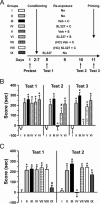
Erasure of CPP does not result from extinction and requires administration of SL327 when animals are reexposed to cocaine in the drug-paired environment. (A) Combined experimental design of the two independent experiments reported in B (groups I-VI) and C (different groups I-IV and groups VII-IX). Each group included eight to nine mice. (B) In addition to groups I-IV, treated as in Fig. 1, groups V and VI received vehicle or SL327 injection, respectively, and 1 h later a saline injection just before being placed in drug-paired compartment. All cocaine-conditioned groups had a significant CPP at test 1 (test 1, F(5,44) = 10.02, P < 0.01). At test 2, CPP was erased in SL327/cocaine-treated group IV and slightly diminished in vehicle/saline-treated group V (partial extinction) (test 2, F(5,44) = 15.43, P < 0.01). For test 3, all mice received a cocaine-priming injection immediately before the test. All cocaine-conditioned mice had a strong CPP, except group IV (test 3, F(5,44) = 13.54, P < 0.01). Data are means ± SEM. Post hoc comparison by Bonferroni test was as follows: group I vs. group II to VI, ∗, P < 0.01; group III vs. group IV, ○, P < 0.01; group II vs. group V, #, P < 0.01. (C) Groups I-IV were treated as above. Groups VII and VIII received a cocaine injection on day 9 in their home cage (HC) preceded by vehicle (group VII) or SL327 (group VIII). Group IX did not receive a cocaine reexposure but was treated with SL327 60 min before test 1. Although group IX displayed a dramatic CPP reduction immediately after SL327 injection, the place preference was blocked only in group IV when animals were tested 2 days later (test 1, F(6,49) = 13.99, P < 0.01; test 2, F(6,49) = 13.78, P < 0.01). Post hoc comparison by Bonferroni test was as follows: group I vs. group II to IV and VII to IX, ∗, P < 0.01; group II vs. group IX, #, P < 0.01; group III vs. group IV, ○, P < 0.01.
Fig. 3.
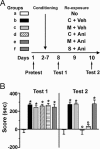
Long-lasting erasure of morphine-CPP by SL327. (A) Experimental design: M, morphine, 5 mg/kg; Veh, vehicle; SL327, 30 mg/kg. Each group included nine mice. (B) After conditioning, mice developed a significant place preference for the morphine-paired side (group II, III, and IV) compared with control group (I) (test 1, F(3,32) = 27.31, P < 0.01). Inhibition of ERK by SL327 during reexposure to morphine (group IV) suppressed place preference when the animals were tested 24 h (test 2, F(3,32) = 25.26, P < 0.01) and 2 weeks later (test 3, F(3,32) = 24.54, P < 0.01). CPP was not restored after a priming injection of morphine at day 25 (test 4, F(3,32) = 13.12, P < 0.01). Data are means ± SEM. Post hoc comparison by Bonferroni test was as follows: group I vs. group II to IV, ∗, P < 0.05; ∗∗, P < 0.01; group III vs. group IV, ○○, P < 0.01; group II vs. group III, ##, P < 0.01.
Fig. 4.
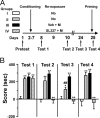
Protein synthesis inhibition after reexposure to morphine or cocaine in the drug-paired compartment erases CPP. (A) Experimental design: C, cocaine 20 mg/kg; M, morphine, 5 mg/kg; Veh, vehicle; Ani, anisomycin, 100 mg/kg. Each group included seven to nine mice. (B) After conditioning, mice developed a significant CPP for the cocaine-paired (groups b, d, and f) or morphine-paired (groups c and e) compartment compared with saline-treated mice (group a) (test 1, F(5,42) = 17.49, P < 0.01). Blockade of protein synthesis after reexposure to cocaine (group d) or morphine (group e) by injection of anisomycin suppressed the place preference when the animals were tested 24 h later (test 2, F(5,42) = 17.47, P < 0.01). Data are expressed as mean ± SEM. Post hoc comparison (Bonferroni test), group a vs. group b–f, was as follows: ∗, P < 0.01; group b or c vs. group d or e, ○, P < 0.01.
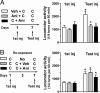
Fig. 5.
Cocaine-induced locomotor sensitization induction is prevented by protein synthesis inhibitors but does not undergo protein synthesis-dependent reconsolidation. (A) Mice received a first injection of cocaine (C, 20 mg/kg), preceded or followed by vehicle (Veh) or anisomycin (Ani, 100 mg/kg) (10–11 mice per group). Locomotor activity was measured during 60 min in response to this first injection (1st inj) of cocaine and to a second injection of cocaine, 7 days later (test inj). Data were analyzed with mixed factor ANOVA (repeated measure over time) (effect of time, F(1,53) = 6.99, P < 0.01; effect of treatment, F(2,53) = 5.36, P < 0.01). Bonferroni test was as follows: ∗, P < 0.01 compared with 1st inj; ○, P < 0.01 Veh vs. Ani. (B) To test possible reconsolidation process, all groups of mice received a 1st injection of cocaine (1st inj). Three days later, they were left untreated (no reexposure) or received a second injection of cocaine in the actimeter, followed by an injection of vehicle (C + Veh) or anisomycin (C + Ani). All mice received a test injection of cocaine at day 7 (test inj) (10 mice per group). Data were analyzed with mixed factor ANOVA (repeated measure over time) (effect of time, F(1,53) = 35.82, P < 0.01; effect of treatment, F(2,53) = 0.26, NS). Bonferroni test was as follows: ∗, P < 0.01 compared with 1st inj.
Similar articles
- The MEK inhibitor SL327 blocks acquisition but not expression of lithium-induced conditioned place aversion: a behavioral and immunohistochemical study.
Longoni R, Spina L, Vinci S, Acquas E. Longoni R, et al. Psychopharmacology (Berl). 2011 Jul;216(1):63-73. doi: 10.1007/s00213-011-2192-9. Epub 2011 Feb 11. Psychopharmacology (Berl). 2011. PMID: 21312031 - Role of the ERK pathway in psychostimulant-induced locomotor sensitization.
Valjent E, Corvol JC, Trzaskos JM, Girault JA, Hervé D. Valjent E, et al. BMC Neurosci. 2006 Mar 2;7:20. doi: 10.1186/1471-2202-7-20. BMC Neurosci. 2006. PMID: 16512905 Free PMC article. - Inhibition of extracellular signal-regulated kinase (ERK) activity with SL327 does not prevent acquisition, expression, and extinction of ethanol-seeking behavior in mice.
Groblewski PA, Franken FH, Cunningham CL. Groblewski PA, et al. Behav Brain Res. 2011 Mar 1;217(2):399-407. doi: 10.1016/j.bbr.2010.11.018. Epub 2010 Nov 11. Behav Brain Res. 2011. PMID: 21074569 Free PMC article. - Drug-sensitive reward in crayfish: an invertebrate model system for the study of SEEKING, reward, addiction, and withdrawal.
Huber R, Panksepp JB, Nathaniel T, Alcaro A, Panksepp J. Huber R, et al. Neurosci Biobehav Rev. 2011 Oct;35(9):1847-53. doi: 10.1016/j.neubiorev.2010.12.008. Epub 2010 Dec 21. Neurosci Biobehav Rev. 2011. PMID: 21182861 Free PMC article. Review. - Drug-induced alterations in the extracellular signal-regulated kinase (ERK) signalling pathway: implications for reinforcement and reinstatement.
Zhai H, Li Y, Wang X, Lu L. Zhai H, et al. Cell Mol Neurobiol. 2008 Feb;28(2):157-72. doi: 10.1007/s10571-007-9240-3. Epub 2007 Nov 28. Cell Mol Neurobiol. 2008. PMID: 18041576 Free PMC article. Review.
Cited by
- Contributions of extracellular-signal regulated kinase 1/2 activity to the memory trace.
Ojea Ramos S, Feld M, Fustiñana MS. Ojea Ramos S, et al. Front Mol Neurosci. 2022 Oct 5;15:988790. doi: 10.3389/fnmol.2022.988790. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36277495 Free PMC article. Review. - Conditioned drug reward enhances subsequent spatial learning and memory in rats.
Zhai HF, Zhang ZY, Zhao M, Qiu Y, Ghitza UE, Lu L. Zhai HF, et al. Psychopharmacology (Berl). 2007 Dec;195(2):193-201. doi: 10.1007/s00213-007-0893-x. Epub 2007 Jul 28. Psychopharmacology (Berl). 2007. PMID: 17661018 - Memory reconsolidation in aversive and appetitive settings.
Reichelt AC, Lee JL. Reichelt AC, et al. Front Behav Neurosci. 2013 Sep 9;7:118. doi: 10.3389/fnbeh.2013.00118. Front Behav Neurosci. 2013. PMID: 24058336 Free PMC article. Review. - The NMDA antagonist MK-801 disrupts reconsolidation of a cocaine-associated memory for conditioned place preference but not for self-administration in rats.
Brown TE, Lee BR, Sorg BA. Brown TE, et al. Learn Mem. 2008 Dec 2;15(12):857-65. doi: 10.1101/lm.1152808. Print 2008 Dec. Learn Mem. 2008. PMID: 19050157 Free PMC article. - The MEK inhibitor SL327 blocks acquisition but not expression of lithium-induced conditioned place aversion: a behavioral and immunohistochemical study.
Longoni R, Spina L, Vinci S, Acquas E. Longoni R, et al. Psychopharmacology (Berl). 2011 Jul;216(1):63-73. doi: 10.1007/s00213-011-2192-9. Epub 2011 Feb 11. Psychopharmacology (Berl). 2011. PMID: 21312031
References
- Schultz W., Dayan P., Montague P. R. Science. 1997;275:1593–1599. - PubMed
- Reynolds J. N. J., Wickens J. R. Neural Netw. 2002;15:507–521. - PubMed
- Hyman S. E., Malenka R. C. Nat. Rev. Neurosci. 2001;2:695–703. - PubMed
- Nestler E. J. Neuropharmacology. 2004;47(Suppl. 1):24–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous