Recruitment of ATR to sites of ionising radiation-induced DNA damage requires ATM and components of the MRN protein complex - PubMed (original) (raw)
Recruitment of ATR to sites of ionising radiation-induced DNA damage requires ATM and components of the MRN protein complex
K E Adams et al. Oncogene. 2006.
Abstract
ATM and ATR are two related kinases essential for signalling DNA damage. Although ATM is thought to be the principle kinase responsible for signalling ionising radiation (IR)-induced DNA damage, ATR also contributes to signalling this form of genotoxic stress. However, the molecular basis of differential ATM and ATR activation in response to IR remains unclear. Here, we report that ATR is recruited to sites of IR-induced DNA damage significantly later than activation of ATM. We show that ATR is recruited to IR-induced nuclear foci in G(1) and S phase of the cell cycle, supporting a role for ATR in detecting DNA damage outside of S phase. In addition, we report that recruitment of ATR to sites of IR-induced DNA damage is concomitant with appearance of large tracts of single-stranded DNA (ssDNA) and that this event is dependent on ATM and components of the Mre11/Rad50/Nbs1 (MRN) protein complex.
Figures
Figure 1
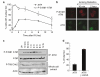
IR-induced ATR nuclear retained foci become apparent at times after activation of ATM. (a) HeLa cells were left untreated or treated with 0.5 Gy IR, and subjected to immunofluorescence using P-S1981 ATM or ATR antibodies at time points following irradiation as indicated. The number of cells displaying nuclear foci was scored at each time point from a population of ≥200 cells and error bars represent the s.e.m. (standard error of the mean). (b) Representative pictures of cells described in (a). (c) HeLa cells were left untreated (un.), or exposed to 0.5 Gy of IR and whole-cell extracts prepared at the indicated time points following administration of DNA damage. Immunoprecipitation of ATM was performed before Western blotting with either P-S1981 ATM or ATM antibodies as indicated. In parallel, Western blots on whole-cell extracts were also performed using antibodies specific for either actin or Chk2 phosphorylated on T68 (P-T68 Chk2). (d). HeLa cells were exposed to 0.5 Gy of IR and costained with γ-H2AX and ATR antibodies 2 h after administration of DNA damage. The % of ATR foci that do (ATR/γ-H2AX) or do not (ATR) colocalise with γ-H2AX was scored from ≥200 ATR foci. Error bars represent the s.e.m.
Figure 2
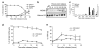
IR-induced ATR foci are apparent in both G1 and S phase of the cell cycle. (a) Swiss 3T3 cells were released from quiescence and allowed to progress through the cell cycle. Samples were taken at the time points indicated and the percentage of cells in each phase of the cell cycle established by FACS analysis. Error bars represent the s.e.m. (b) Chromatin extracts were prepared from either asynchronous cells (Asy.), quiescent cells (Go), or cells released from quiescence as described in (a). Equivalent amounts of extracts were subjected to Western blotting using antibodies as indicated. (c) Cells described in (a) were subjected to immunofluorescence using an antibody specific for PCNA. The number of cells in early, mid or late S phase was established by scoring nuclear PCNA staining patterns (Celis and Celis, 1985). Error bars represent the s.e.m. (d and e) Swiss 3T3 cells described in (a) were left either untreated or exposed to 0.5 Gy IR (+IR) at the time points indicated after release from quiescence. Cells were allowed 2 h recovery before being subjected to immunofluorescence with antibodies against either P-S1981 ATM (d) or ATR (e). A population of ≥ 200 cells was scored at each time point and error bars represent the s.e.m.
Figure 3
Overexpression of dominant-negative kinase-dead ATR results in sensitivity to IR administered during G1 phase of the cell cycle. (a and b) U2OS cells were uninduced (−) or induced (+) to express either wild-type (WT) or kinase-dead (KD) ATR for 48 h and the distribution of cells throughout the cell cycle established by FACS (a). Cells were treated with increasing doses of IR and left to recover for 7–10days. Survival of cells was assessed by scoring colonies containing ≥ 50cells. The number of colonies at each IR dose is expressed as a percentage of the untreated control and error bars represent the s.e.m. (b). (c and d) U2OS cells were uninduced (−) orinduced (+) to express either wild-type (WT) or kinase-dead (KD) ATR for 48 h before synchronisation of cells in the G1 by release from a nocodazole block. Synchrony of cell cultures was established by FACS analysis (c). Cell survival following administration of IR 6 h after release from nocodazole (d) was assessed as in b.
Figure 4
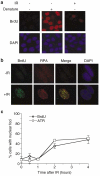
IR-induced ATR foci formation is concomitant with formation of large tracts of ssDNA in situ. (a) After incorporation of BrdU into their genome (see Materials and methods), asynchronous HeLa cells were exposed to 0.5 Gy IR as indicated. Cells were fixed 2 h after IR and subjected to immunofluorescence with BrdU antibodies. To demonstrate incorporation of BrdU into the genome, a sample was included in which cellular DNA was denatured with 0.2 M HCl before immunofluorescence. (b) Asynchronous HeLa cells were treated as in (a) and subjected to immunofluorescence with RPA34 and BrdU antibodies. (c) Asynchronous HeLa cells were treated as in (a) and subjected to immunofluorescence with the indicated antibodies at time points after administration of IR. Cells were scored for nuclear foci from a population of ≥ 200 cells and error bars represent the s.e.m.
Figure 5
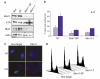
Components of the MRN complex are required for ATR foci formation following administration of IR. (a) Asynchronous HeLa cells were left either untreated (Non), or transfected with nontargeting siRNA (NT) or siRNA complementary to two distinct regions of Mre11 (Mre11-SP or Mre11-1). Cells were harvested 72 h after transfection and subjected to Western blotting with the indicated antibodies. (b) Asynchronous HeLa cells were transfected with nontargeting siRNA (NT) or Mre11 siRNA as indicated. At 72 h after transfection, cells were left untreated or treated with 0.5 Gy IR, and after 2 h recovery subjected to immunofluorescence with ATR antibodies. Samples were scored for cells that displayed nuclear retained ATR foci from a population of ≥ 200 cells and error bars represent the s.e.m. (c) Representative pictures of cells described in (b). (d) FACS analysis representing cell cycle distribution of cells 72 h after transfection of cells with either nontargeting or Mre11 (Mre11-SP and Mre11-1) siRNA oligonucleotides.
Figure 6
ATM is required for ATR foci formation and ATR-mediated signalling to Chk1 following administration of IR. (a and b) Asynchronous HeLa cells were either preincubated in 10 μ
m
ATM inhibitor (KU-55933 (+ ATMi)) or left in normal growth media (− ATMi) for 1 h before exposure to 0.5 Gy of IR. Untreated cells (un.), or cells that had been exposed to IR were fixed either 2 h (2 h), 4 h (4 h), or 8 h (8 h) after irradiation and stained for either P-S1981 ATM (A) or ATR (B). Cells with nuclear foci were scored from a population of greater ≥200 cells. Error bars represent the s.e.m. (c) Asynchronous HeLa cells were transfected with either nontargeting siRNA oligonucleotide (NT), or siRNA oligonucleotide complementary to ATR (ATR2) as described in Materials and methods and processed 72 h after the first transfection. Western blots were performed on whole-cell extracts with the indicated antibodies (left hand panel). In parallel, cells were left untreated, or exposed to 0.5 Gy of IR and stained for either P-S1981 ATM or ATR antibodies 2 h after irradiation as indicated. (d) Asynchronous HeLa cells were transfected with either nontargeting siRNA oligonucleotide (NT), or siRNA oligonucleotide complementary to ATR (ATR2) and 72 h after the first transfection left untreated, or exposed to 10Gy IR as indicated. Whole-cell extracts were prepared 2 h after administration of DNA damage and subjected to Western blotting using antibodies as indicated. (e) Asynchronous HeLa cells were either preincubated in 10 μ
m
ATM inhibitor (KU-55933 (+ ATMi)) or left in normal growth media (− ATMi) for 1 h before exposure to 10Gy of IR. Whole-cell extracts were prepared 2 h after administration of DNA damage and subjected to Western blotting using antibodies as indicated.
Similar articles
- Ataxia-telangiectasia-like disorder (ATLD)-its clinical presentation and molecular basis.
Taylor AM, Groom A, Byrd PJ. Taylor AM, et al. DNA Repair (Amst). 2004 Aug-Sep;3(8-9):1219-25. doi: 10.1016/j.dnarep.2004.04.009. DNA Repair (Amst). 2004. PMID: 15279810 Review. - Ataxia telangiectasia-mutated (ATM) kinase activity is regulated by ATP-driven conformational changes in the Mre11/Rad50/Nbs1 (MRN) complex.
Lee JH, Mand MR, Deshpande RA, Kinoshita E, Yang SH, Wyman C, Paull TT. Lee JH, et al. J Biol Chem. 2013 May 3;288(18):12840-51. doi: 10.1074/jbc.M113.460378. Epub 2013 Mar 22. J Biol Chem. 2013. PMID: 23525106 Free PMC article. - A role for the MRN complex in ATR activation via TOPBP1 recruitment.
Duursma AM, Driscoll R, Elias JE, Cimprich KA. Duursma AM, et al. Mol Cell. 2013 Apr 11;50(1):116-22. doi: 10.1016/j.molcel.2013.03.006. Mol Cell. 2013. PMID: 23582259 Free PMC article. - The Mre11-Rad50-Nbs1 complex mediates activation of TopBP1 by ATM.
Yoo HY, Kumagai A, Shevchenko A, Shevchenko A, Dunphy WG. Yoo HY, et al. Mol Biol Cell. 2009 May;20(9):2351-60. doi: 10.1091/mbc.e08-12-1190. Epub 2009 Mar 11. Mol Biol Cell. 2009. PMID: 19279141 Free PMC article. - The role of NBS1 in the modulation of PIKK family proteins ATM and ATR in the cellular response to DNA damage.
Zhou J, Lim CU, Li JJ, Cai L, Zhang Y. Zhou J, et al. Cancer Lett. 2006 Nov 8;243(1):9-15. doi: 10.1016/j.canlet.2006.01.026. Epub 2006 Mar 10. Cancer Lett. 2006. PMID: 16530324 Free PMC article. Review.
Cited by
- Loss of ATM kinase activity leads to embryonic lethality in mice.
Daniel JA, Pellegrini M, Lee BS, Guo Z, Filsuf D, Belkina NV, You Z, Paull TT, Sleckman BP, Feigenbaum L, Nussenzweig A. Daniel JA, et al. J Cell Biol. 2012 Aug 6;198(3):295-304. doi: 10.1083/jcb.201204035. J Cell Biol. 2012. PMID: 22869595 Free PMC article. - Dual role for Saccharomyces cerevisiae Tel1 in the checkpoint response to double-strand breaks.
Mantiero D, Clerici M, Lucchini G, Longhese MP. Mantiero D, et al. EMBO Rep. 2007 Apr;8(4):380-7. doi: 10.1038/sj.embor.7400911. Epub 2007 Mar 9. EMBO Rep. 2007. PMID: 17347674 Free PMC article. - DNA methylation inhibitor 5-Aza-2'-deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B.
Palii SS, Van Emburgh BO, Sankpal UT, Brown KD, Robertson KD. Palii SS, et al. Mol Cell Biol. 2008 Jan;28(2):752-71. doi: 10.1128/MCB.01799-07. Epub 2007 Nov 8. Mol Cell Biol. 2008. PMID: 17991895 Free PMC article. - Ataxia telangiectasia and Rad3-related inhibitors and cancer therapy: where we stand.
Mei L, Zhang J, He K, Zhang J. Mei L, et al. J Hematol Oncol. 2019 Apr 24;12(1):43. doi: 10.1186/s13045-019-0733-6. J Hematol Oncol. 2019. PMID: 31018854 Free PMC article. Review. - Double-strand breaks in ribosomal RNA genes activate a distinct signaling and chromatin response to facilitate nucleolar restructuring and repair.
Korsholm LM, Gál Z, Lin L, Quevedo O, Ahmad DA, Dulina E, Luo Y, Bartek J, Larsen DH. Korsholm LM, et al. Nucleic Acids Res. 2019 Sep 5;47(15):8019-8035. doi: 10.1093/nar/gkz518. Nucleic Acids Res. 2019. PMID: 31184714 Free PMC article.
References
- Abraham RT. Genes Dev. 2001;15:2177–2196. - PubMed
- Bakkenist CJ, Kastan MB. Nature. 2003;421:499–506. - PubMed
- Banin S, Moyal L, Shieh SY, Taya Y, Anderson CW, Chessa L, et al. Science. 1998;281:1674–1677. - PubMed
- Bartek J, Lukas J. Cancer Cell. 2003;3:421–429. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous