Huntingtin-HAP40 complex is a novel Rab5 effector that regulates early endosome motility and is up-regulated in Huntington's disease - PubMed (original) (raw)
Huntingtin-HAP40 complex is a novel Rab5 effector that regulates early endosome motility and is up-regulated in Huntington's disease
Arun Pal et al. J Cell Biol. 2006.
Abstract
The molecular mechanisms underlying the targeting of Huntingtin (Htt) to endosomes and its multifaceted role in endocytosis are poorly understood. In this study, we have identified Htt-associated protein 40 (HAP40) as a novel effector of the small guanosine triphosphatase Rab5, a key regulator of endocytosis. HAP40 mediates the recruitment of Htt by Rab5 onto early endosomes. HAP40 overexpression caused a drastic reduction of early endosomal motility through their displacement from microtubules and preferential association with actin filaments. Remarkably, endogenous HAP40 was up-regulated in fibroblasts and brain tissue from human patients affected by Huntington's disease (HD) as well as in STHdhQ(111) striatal cells established from a HD mouse model. These cells consistently displayed altered endosome motility and endocytic activity, which was restored by the ablation of HAP40. In revealing an unexpected link between Rab5, HAP40, and Htt, we uncovered a new mechanism regulating cytoskeleton-dependent endosome dynamics and its dysfunction under pathological conditions.
Figures
Figure 1.
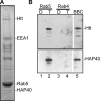
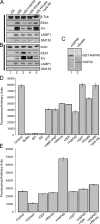
Htt and HAP40 elute from immobilized Rab5. (A) SDS-PAGE of proteins eluted from immobilized GST-Rab5 that was loaded with GTPγS. The indicated bands were found to correspond to Htt, HAP40, Rab5-GST, and EEA1 by mass spectrometry analysis. (B) Western blot analysis of chromatographic eluates from GST-Rab5 (lanes 1 and 2) and -Rab4 (lanes 3 and 4) affinity columns preloaded with GDP (D; lanes 1 and 3) or GTPγS (T; lanes 2 and 4). The bovine brain cytosol (BBC; lane 5) was used as a source of proteins. Blots were probed for Htt and HAP40 as indicated.
Figure 2.
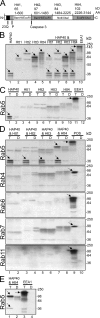
Htt–HAP40 complex is a novel Rab5 effector. (A) Schematic 23 glutamine residues (23Q), the adjacent proline-rich sequence (P), the caspase-3 cleavage site, and the four recombinant fragments (Htt1–4) cloned in pcDNA3.1 using the indicated restriction sites. (B) Autoradiograph of in vitro–translated Htt fragments (Htt1–4) and HAP40. Full-length HAP40 cDNA and Htt fragments were in vitro translated in the presence of [35S]methionine, separated by SDS-PAGE, and autoradiographed. Given their large size, the bands of strongest intensity (arrows) and corresponding to the predicted masses for HAP40 (∼40 kD, lane 1), Htt1 (∼65 kD, lane 2), Htt2 (∼97 kD, lane 3), Htt3 (∼84 kD, lane 4), and Htt4 (∼102 kD, lane 5) were accompanied by multiple products because of either initiation of translation at internal sites or premature termination. Lanes 6–9: each Htt fragment was cotranslated with HAP40. (C) Autoradiograph of the in vitro–translated proteins in B eluted from immobilized GST-Rab5 preloaded with GTPγS (T) or GDP (D). (D) Autoradiograph of Htt fragments cotranslated in vitro with HAP40 and eluted from various immobilized Rab proteins as indicated. The experiment was performed as described in C, but Htt fragments and HAP40 were cotranslated (see B, lanes 6–9) and applied onto immobilized Rab proteins. Besides HAP40, the COOH-terminal Htt fragment (Htt4) was eluted from GST-Rab5 beads (compare lanes 7 with 8), whereas Htt1 (lanes 1 and 2), Htt2 (lanes 3 and 4), and Htt3 (lanes 5 and 6) did not show significant specific association with Rab5. Moreover, none of the Htt fragments or HAP40 displayed specific interactions with GST-Rab4, 6, 7, or 11. Positive controls (POS, lanes 9 and 10) for each Rab protein were EEA1 (∼170 kD) for Rab5, Rabenosyn-5 (∼89 kD) for Rab4 (de Renzis et al., 2002), VPS39 (∼100 kD) for Rab7 (Rink et al., 2005), and GapCenA (∼115 kD) for Rab6 and 11 (Cuif et al., 1999 and unpublished data). (E) Htt and HAP40 elute in equimolar amounts from the Rab5 column. A mixture of Htt4 and HAP40 was applied onto Rab5 columns and eluted as in D but with a 10-fold excess of Htt4 to prevent HAP40 from binding freely to Rab5. (C–E) Arrows point to correct translation products as in B.
Figure 3.
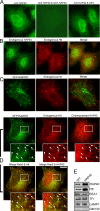
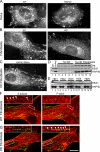
Overexpressed HAP40 recruits Htt onto early endosomes. Immunofluorescence microscopy analysis of HeLa cells expressing EGFP-Rab5 and/or HAP40 and immunostained for HAP40 and Htt as indicated. (A) The anti-HAP40 antibody specifically recognizes its antigen (green). Cells were stained with the antibody alone (anti-HAP40) or premixed with HAP40-GST fusion (anti-HAP40 & GST-HAP40) or GST protein (anti-HAP40 & GST). The image after specific depletion of the antibody (anti-HAP40 & GST-HAP40) was obtained at sixfold prolonged exposure time compared with the others. (B) Endogenous Htt (red) and endogenous HAP40 (green); <1% overlapping (n = 10). (C) Endogenous Htt (red) and EGFP-Rab5 (green); 7% overlapping (SD ± 5%, n = 10). (D) Overexpressed HAP40 (red) recruits endogenous Htt (red) onto endosomes labeled with EGFP-Rab5WT (green); 43% overlapping of endogenous Htt with EGFP-Rab5 (merge Rab5 & Htt; SD ± 6%, n = 10) and 31% overlapping of overexpressed HAP40 with EGFP-Rab5 (merge Rab5 & HAP40; SD ± 7%, n = 10). Cells were cotransfected with HAP40 and GFP-Rab5WT expression constructs. Arrowheads in insets (magnified images of boxed areas) highlight colocalization. Bar, 10 μm. (E) Western blot analysis of early endosomes prepared from untreated (WT) or HeLa cells overexpressing HAP40 (HAP40). Blots were probed for HAP40, Htt, EEA1, transferrin receptor (Tfr), LAMP1, and GM130 as indicated.
Figure 4.
Htt is recruited onto early endosomes in a Rab5- and HAP40-dependent fashion. Immunofluorescence microscopy analysis of HeLa cells transfected with expression vectors for EGFP-Rab5Q79L alone or cotransfected with siRNA duplexes against HAP40 as indicated. (A) EGFP-Rab5Q79L (green) recruits endogenous Htt (red) onto endosomes; 52% overlapping (SD ± 7%, n = 8). Cells were transfected with EGFP-Rab5Q79L expression construct alone. (B) RNAi against HAP40 (siHAP40) leads to the loss of endogenous Htt (red) from endosomes; 8% overlapping (SD ± 6%, n = 9). Cells were cotransfected with HAP40 siRNA and EGFP-Rab5Q79L (green) expression constructs. Bar, 10 μm. (C) Knockdown of HAP40 protein by RNAi as shown by Western blot analysis. EEA1 and HAP40 expression levels in untransfected cells (control) and cells transfected with unrelated (siGFP) or siRNA against HAP40 (siHAP40).
Figure 5.
Htt and HAP40 decrease in vitro reconstituted motility of early endosomes along microtubules. (A) Purified early endosomes labeled by internalization of rhodamine–transferrin were mixed with buffer (control) alone or with various eluates of bovine brain cytosol proteins that were affinity purified on GST-Rab5 columns. Eluate obtained from columns loaded with GDP was added directly to the sample (+GDP-eluate). Eluate obtained from columns loaded with GTPγS was added directly to the sample (+GTP-eluate) or after preincubation with antiserum against the cytoplasmic domain of Tfr (+eluate + anti-Tfr), antiserum against Htt (+eluate + anti-Htt), or without eluate but with 1 μM of recombinant GST-HAP40 fusion protein (+HAP40). In vitro motility of early endosomes along microtubules recorded using time-lapse fluorescence video microscopy (see Materials and methods) was quantified by counting motility events per video. Error bars show the SD of 10 videos. (B) Videos recorded under control conditions (control) or with 1 μM GST-HAP40 fusion protein (HAP40) used for the analysis in A are displayed as merged stacks of overlaid images collected at 2-s intervals over 2 min. When represented in this manner, a moving object will generate a trajectory consisting of a linear series of overlapping spots. Bar, 10 μm.
Figure 6.
Modulation of binding of early endosomes to microtubules and F-actin by Htt and HAP40. (A) Reduction of binding of early endosomes (EE) to microtubules in vitro caused by Htt and HAP40. A spin-down assay was performed (see Materials and methods), and the resulting pellet of microtubule-associated material was analyzed by immunoblotting with antibodies against proteins indicated on the right. (B) Stimulation of binding of early endosomes to F-actin in vitro caused by Htt and HAP40. A spin-down assay was performed as described for A but with 10 μg of freshly polymerized F-actin replacing the microtubule. (A and B) Microtubules (A) or F-actin (B) spun alone (−EE, lane 1), with early endosomes (control; +EE, lane 2), with early endosomes and 1 μM GST-HAP40 fusion protein (+EE+HAP40, lane 3), with early endosomes and 10 μg GST-Rab5–GTPγS column eluate (+EE+eluate, lane 4), or eluate preincubated with anti-Htt antiserum (+EE+eluate+anti-Htt, lane 5). (C) Comparison of HAP40 protein levels in samples prepared for the early endosome–microtubule/actin-binding assays. Samples were prepared as described for A and B either with GST-HAP40 fusion (lane 1) or GST-Rab5–GTPγS column eluate (lane 2). 10-μl aliquots of each sample were separated by SDS-PAGE and immunoblotted for HAP40. Bands corresponded to the correct masses of GST-HAP40 or HAP40 as indicated on the right. Nonrelevant lanes on the same blot were sliced out in Adobe Photoshop to juxtapose the lanes shown. (D) Quantifications of early endosomes bound to microtubules. Binding was performed as described for A, but the resulting pellet of microtubule-associated material was lysed to release the rhodamine–transferrin label of early endosome. Fluorescence (arbitrary units) served as a direct measure for the amount of early endosomes bound to microtubules. Error bars represent the SD of samples in triplicate. Binding was performed in the presence of 15 μg of early endosomes and 16 μg of microtubules (control), in the absence of microtubules (−MT), in the absence of early endosomes (−EE), in the absence of ATP (−ATP), with all three components omitted (buffer), with 2 mM adenylyl-imidodiphosphate (+AMP-PNP), with 1 μM of recombinant RN-tre (+RN-tre), with 1 μM Rab-GDI (+GDI), with 1 μM HAP40-GST fusion protein (+HAP40), with 1 μM GST (+GST), with 10 μg GST-Rab5–GTPγS column eluate (+eluate), or with eluate preincubated with anti-Htt antiserum (+eluate+anti-Htt). (E) Quantifications of early endosomes bound to F-actin. Binding was performed as described for B, and quantifications were made as in D. Binding was performed in the presence of 15 μg of early endosomes, 10 μg of F-actin (control), and with the addition of various components as in D.
Figure 7.
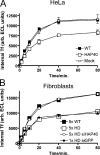
Elevated protein levels of HAP40 shift early endosomes from microtubules to actin filaments, causing a severe decrease of motility in vivo. HeLa cells and human primary fibroblasts expressing EGFP-Rab5 were imaged using time-lapse video microscopy. (A–C) Images generated by merging a stack of overlaid images collected at 300-ms intervals over 2 min, as for Fig. 5 B. Videos corresponding to A–C are available as online supplemental materials (Videos 1–5, available at
http://www.jcb.org/cgi/content/full/jcb.200509091/DC1
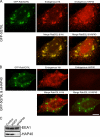
). (A) In HeLa cells, coexpression of EGFP-Rab5 and HAP40 (right) led to a drastic reduction in endosome motility compared with EGFP-Rab5 alone (WT, left). (B) In fibroblasts from HD patients (HD), such reduction was also evident from the comparison with fibroblasts from healthy individuals (WT). (C) RNAi against HAP40 in these HD fibroblasts (HAP40 RNAi) restores endosome motility. HD fibroblast cell lines were cotransfected with EGFP-Rab5 expression vector and siRNA against HAP40. (D) Similar to HeLa cells overexpressing HAP40 (compare lane 1 with lane 2), endogenous protein levels of HAP40 were elevated in five HD fibroblast cell lines (lanes 8–12) compared with fibroblasts from five healthy individuals (lanes 3–7). Blots were also probed with anti-EEA1 and anti-Htt antiserum to confirm equal loading and the identity of the HD cell lines (see Results). (E) Knockdown of HAP40 protein levels in fibroblasts from all five HD cell lines by RNAi. Western blot analysis of EEA1 and HAP40 in untransfected cells (lanes 1, 4, 7, 10, and 13), cells transfected with unrelated siRNA against GFP (lanes 2, 5, 8, 11, and 14), or HAP40 siRNA (lanes 3, 6, 9, 12, and 15). (F) EGFP-Rab5–labeled endosomes align primarily with microtubules in healthy fibroblasts. Primary human fibroblasts from healthy individuals were transfected with EGFP-Rab5 (green) and fixed and immunostained for microtubules and F-actin. The same cell is shown with its β-tubulin staining (red) on the left and for F-actin (red) on the right, as indicated. Arrowheads in the insets (magnifications of the boxed areas) point to endosomes aligned with microtubules. Overlap of EGFP-Rab5 with tubulin signals was 82% (SD ± 9%, n = 10) for healthy fibroblasts and 15% (SD ± 6%, n = 9) for HD cell lines (see G). (G) The cell processed as in F shows that EGFP-Rab5–labeled endosomes align strikingly with F-actin in fibroblasts from HD patients. Arrowheads in the insets point to endosomes (green) aligned with F-actin (red). Overlap of EGFP-Rab5 with actin was 2% (SD ± 1%, n = 10) for healthy fibroblasts (see F) and 44% (SD ± 8%, n = 10) for HD cell lines. Bar, 10 μm.
Figure 8.
Elevated endogenous HAP40 protein levels impair Rab5 dynamics in vivo in STHdhQ111/111 and STHdhQ7/111 striatal cells from a HD mouse model. (A) Cells expressing normal (STHdhQ7/7) or mutant (STHdhQ111/111 and STHdhQ7/111) Htt were transfected with EGFP-Rab5 plasmid, differentiated, and imaged using time-lapse video microscopy. Overlaid images were generated as described for Fig. 5 B. Videos corresponding to A are available as online supplemental material (Videos 6–8, available at
http://www.jcb.org/cgi/content/full/jcb.200509091/DC1
). Motility of Rab5 compartments was drastically reduced in STHdhQ111/111 and STHdhQ7/111 compared with STHdhQ7/7 cells. Insets show magnifications of boxed areas. Bar, 10 μm. (B) Endogenous protein levels of HAP40 are elevated in STHdhQ111/111 (lane 1) and STHdhQ7/111 (lane 2) compared with STHdhQ7/7 cells (lane 3) as well as in striatal tissue from five human postmortem brains affected by HD (lanes 4–8) compared with healthy control brains (lanes 9–13). Blots were also probed with anti-EEA1 and anti-Htt antiserum to confirm equal loading and the expression of wild-type and mutant Htt as described for Fig. 7 D.
Figure 9.
Htt and HAP40 are recruited onto Rab5 vesicles in primary fibroblasts from human HD patients and STHdhQ7/111 striatal cells from a HD mouse model. (A) Image gallery of the same fibroblast cell from a healthy individual transfected with EGFP-Rab5 (green) and fixed and immunostained for endogenous HAP40 (red) and Htt (red). Boxed areas are magnified in the top panels. (B) The same analysis of a fibroblast from a HD patient. Yellow (arrowheads) indicates colocalization of EGFP-Rab5 and Htt (merge Rab5 and Htt,) or of EGFP-Rab5 and HAP40 (merge Rab5 and HAP40). Overlapping of EGFP-Rab5 with Htt was 6% (SD ± 3%, n = 10) for fibroblasts from healthy individuals (A) and 46% (SD ± 8%, n = 10) from HD patients (B). Overlapping of EGFP-Rab5 with HAP40 was 4% (SD ± 3%, n = 10) for fibroblasts from healthy individuals (A) and 51% (SD ± 9%, n = 10) from HD patients (B). (C) Image gallery of the same differentiated STHdhQ7/7 cell (homozygous for normal Htt) transfected with EGFP-Rab5 (green) and immunostained for endogenous HAP40 (red) and Htt (red). (D) The same analysis of a differentiated STHdhQ7/111 cell (heterozygous for mutant Htt). Overlapping of EGFP-Rab5 with Htt was 5% (SD ± 4%, n = 10) for STHdhQ7/7 cells (C) and 83% (SD ± 12%, n = 10) for STHdhQ7/111 cells (D). Overlapping of EGFP-Rab5 with HAP40 was 4% (SD ± 3%, n = 10) for STHdhQ7/7 cells (C) and 86% (SD ± 15%, n = 10) for STHdhQ7/111 cells (D). The analysis was restricted to cellular outgrowths. Essentially the same results were obtained for STHdhQ111/111 cells (homozygous for mutant Htt; not depicted). Bar, 10 μm.
Figure 10.
Elevated levels of HAP40 reduces transferrin uptake. (A) HeLa cells transfected with HAP40 expression (HAP40), empty plasmid (mock), or untreated (WT) were serum starved, allowed to internalize biotinylated transferrin (Tf) for the indicated times, washed, and lysed, and internalized transferrin was quantified. The mean values of triplicate samples from one representative experiment out of three with SD (error bars, often omitted by plot symbols) are shown. (B) The same uptake for primary human fibroblasts untreated or transfected with siRNA duplexes against HAP40 (siHAP40) or unrelated GFP (siGFP). The mean values of different cell lines from five healthy persons (5× WT), five HD patients (5× HD), the same but treated with siHAP40 (5× HD siHAP40), or the mean values of triplicate sample obtained from one HD line treated with unrelated siGFP (1× HD siGFP) are shown. Error bars (mostly omitted by plot symbols) represent the SD between cell lines (5× WT, 5× HD, and 5× HD siHAP40) or of triplicate samples (1× HD siGFP).
Similar articles
- Huntingtin and Its Partner Huntingtin-Associated Protein 40: Structural and Functional Considerations in Health and Disease.
Seefelder M, Klein FAC, Landwehrmeyer B, Fernández-Busnadiego R, Kochanek S. Seefelder M, et al. J Huntingtons Dis. 2022;11(3):227-242. doi: 10.3233/JHD-220543. J Huntingtons Dis. 2022. PMID: 35871360 Free PMC article. Review. - Regulation of endosome dynamics by Rab5 and Huntingtin-HAP40 effector complex in physiological versus pathological conditions.
Pal A, Severin F, Höpfner S, Zerial M. Pal A, et al. Methods Enzymol. 2008;438:239-57. doi: 10.1016/S0076-6879(07)38017-8. Methods Enzymol. 2008. PMID: 18413253 - HAP40 is a conserved central regulator of Huntingtin and a potential modulator of Huntington's disease pathogenesis.
Xu S, Li G, Ye X, Chen D, Chen Z, Xu Z, Daniele M, Tambone S, Ceccacci A, Tomei L, Ye L, Yu Y, Solbach A, Farmer SM, Stimming EF, McAllister G, Marchionini DM, Zhang S. Xu S, et al. PLoS Genet. 2022 Jul 19;18(7):e1010302. doi: 10.1371/journal.pgen.1010302. eCollection 2022 Jul. PLoS Genet. 2022. PMID: 35853002 Free PMC article. - Rab5 modulates aggregation and toxicity of mutant huntingtin through macroautophagy in cell and fly models of Huntington disease.
Ravikumar B, Imarisio S, Sarkar S, O'Kane CJ, Rubinsztein DC. Ravikumar B, et al. J Cell Sci. 2008 May 15;121(Pt 10):1649-60. doi: 10.1242/jcs.025726. Epub 2008 Apr 22. J Cell Sci. 2008. PMID: 18430781 Free PMC article. - [Effectors of GTPase Rab5 in endocytosis and signal transduction].
Olchowik M, Miaczyńska M. Olchowik M, et al. Postepy Biochem. 2009;55(2):171-80. Postepy Biochem. 2009. PMID: 19824473 Review. Polish.
Cited by
- Huntingtin Is Required for Epithelial Polarity through RAB11A-Mediated Apical Trafficking of PAR3-aPKC.
Elias S, McGuire JR, Yu H, Humbert S. Elias S, et al. PLoS Biol. 2015 May 5;13(5):e1002142. doi: 10.1371/journal.pbio.1002142. eCollection 2015 May. PLoS Biol. 2015. PMID: 25942483 Free PMC article. - Structure of Membrane-Bound Huntingtin Exon 1 Reveals Membrane Interaction and Aggregation Mechanisms.
Tao M, Pandey NK, Barnes R, Han S, Langen R. Tao M, et al. Structure. 2019 Oct 1;27(10):1570-1580.e4. doi: 10.1016/j.str.2019.08.003. Epub 2019 Aug 26. Structure. 2019. PMID: 31466833 Free PMC article. - Impaired XK recycling for importing manganese underlies striatal vulnerability in Huntington's disease.
Chhetri G, Ke Y, Wang P, Usman M, Li Y, Sapp E, Wang J, Ghosh A, Islam MA, Wang X, Boudi A, DiFiglia M, Li X. Chhetri G, et al. J Cell Biol. 2022 Oct 3;221(10):e202112073. doi: 10.1083/jcb.202112073. Epub 2022 Sep 13. J Cell Biol. 2022. PMID: 36099524 Free PMC article. - Small GTPases of the Rab and Arf Families: Key Regulators of Intracellular Trafficking in Neurodegeneration.
Arrazola Sastre A, Luque Montoro M, Lacerda HM, Llavero F, Zugaza JL. Arrazola Sastre A, et al. Int J Mol Sci. 2021 Apr 23;22(9):4425. doi: 10.3390/ijms22094425. Int J Mol Sci. 2021. PMID: 33922618 Free PMC article. Review. - Huntingtin and Its Partner Huntingtin-Associated Protein 40: Structural and Functional Considerations in Health and Disease.
Seefelder M, Klein FAC, Landwehrmeyer B, Fernández-Busnadiego R, Kochanek S. Seefelder M, et al. J Huntingtons Dis. 2022;11(3):227-242. doi: 10.3233/JHD-220543. J Huntingtons Dis. 2022. PMID: 35871360 Free PMC article. Review.
References
- Block-Galarza, J., K.O. Chase, E. Sapp, K.T. Vaughn, R.B. Vallee, M. DiFiglia, and N. Aronin. 1997. Fast transport and retrograde movement of huntingtin and HAP 1 in axons. Neuroreport. 8:2247–2251. - PubMed
- Chan, E.Y., R. Luthi-Carter, A. Strand, S.M. Solano, S.A. Hanson, M.M. DeJohn, C. Kooperberg, K.O. Chase, M. DiFiglia, A.B. Young, et al. 2002. Increased huntingtin protein length reduces the number of polyglutamine-induced gene expression changes in mouse models of Huntington's disease. Hum. Mol. Genet. 11:1939–1951. - PubMed
- Christoforidis, S., H.M. McBride, R.D. Burgoyne, and M. Zerial. 1999. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 397:621–625. - PubMed
- de Renzis, S., B. Sonnichsen, and M. Zerial. 2002. Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes. Nat. Cell Biol. 4:124–133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous