Abnormal immune response of CCR5-deficient mice to ocular infection with herpes simplex virus type 1 - PubMed (original) (raw)
Abnormal immune response of CCR5-deficient mice to ocular infection with herpes simplex virus type 1
Daniel J J Carr et al. J Gen Virol. 2006 Mar.
Abstract
Ocular herpes simplex virus type 1 (HSV-1) infection elicits a strong inflammatory response that is associated with production of the beta chemokines CCL3 and CCL5, which share a common receptor, CCR5. To gain insight into the role of these molecules in ocular immune responses, the corneas of wild-type (WT) and CCR5-deficient (CCR5-/-) mice were infected with HSV-1 and inflammatory parameters were measured. In the absence of CCR5, the early infiltration of neutrophils into the cornea was diminished. Associated with this aberrant leukocyte recruitment, neutrophils in CCR5-/- mice were restricted to the stroma, whereas in WT mice, these cells trafficked to the stroma and epithelial layers of the infected cornea. Virus titres and cytokine/chemokine levels in the infected tissue of these mice were similar for the first 5 days after infection. However, by day 7 post-infection, the CCR5-/- mice showed a significant elevation in the chemokines CCL2, CCL5, CXCL9 and CXCL10 in the trigeminal ganglion and brainstem, as well as a significant increase in virus burden. The increase in chemokine expression was associated with an increase in the infiltration of CD4 and/or CD8 T cells into the trigeminal ganglion and brainstem of CCR5-/- mice. Surprisingly, even though infected CCR5-/- mice were less efficient at controlling the progression of virus replication, there was no difference in mortality. These results suggest that, although CCR5 plays a role in regulating leukocyte trafficking and control of virus burden, compensatory mechanisms are involved in preventing mortality following HSV-1 infection.
Figures
Figure 1
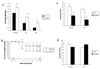
Sensitivity of CCR5-/- mice to HSV-1 infection. C57BL/6 and CCR5-/- mice were infected with 500 pfu/eye of HSV-1 and evaluated for virus titer and the host response to infection. (a) At day 7 post infection, mice (n=11-15/group) were euthanized and the cornea, trigeminal ganglia (TG), and brain stem (BS) were removed using a dissecting microscope, homogenized, and assayed for infectious virus by plaque assay. The data are expressed as the mean pfu (log10) +/- SEM and are pooled from 3-5 experiments with n = 3-4 mice/group/experiment. (b) Mice (n=23-25/group) were monitored and recorded for survival over 30 days post infection. The data are presented as the mean +/- SEM and are pooled from 3 experiments with n=6-10 mice/group/experiment. The difference between survival of infected WT and CCR5-/- mice was significant on day 8 post infection (p<.05). Mice (n=8-16/group) were euthanized at day 3 (c) or day 7 (d) post infection, and the corneas and TG were removed and processed for the composition of CD11b+ cells by flow cytometry. Numbers are in percent population of the gated population +/- SEM. *p < 0.05 for comparison of the WT to CCR5-/- mice.
Figure 2
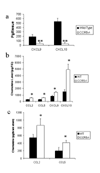
(a) CCR5-/- mice express less CXCL9 and CXCL10 in the TG day 3 post infection. WT C57BL/6 and CCR5-/- mice (n = 8 mice/group for CXCL9 and n = 15/group for CXCL10) were infected with 500 pfu/eye HSV-1. At day 3 post infection, the mice were euthanized and the trigeminal ganglia (TG) were removed and assayed for the indicated chemokine or cytokine by ELISA. The data are expressed as the mean +/- SEM and are pooled from 2-4 experiments with n = 3-4 mice/group/experiment. Chemokine/cytokine expression in the trigeminal ganglia (TG) (b) and brain stem (c) of HSV-1 infected mice. Mice (n=8-15/group) were infected with 500 pfu/eye HSV-1 and euthanized 7 days post infection. The TG and brain stems were removed, homogenized, and assayed for cytokine/chemokine levels by ELISA. Bars represent mean +/- SEM reported in pg/TG (b) and pg/brain stem (c) for each analyte taken from 4-5 mice/group/experiment. **p<.01, *p<.05 comparing the C57BL/6 to CCR5-/- groups.
Figure 3
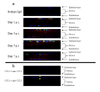
Composite whole mount staining of corneas for CCL5 and HSV-1 antigen expression prior to and after virus infection. WT C57BL/6 and CCR5-/- mice were infected with 500 pfu/eye HSV-1. (a) Mice were euthanized at the indicated time post infection (p.i.) and inspected for virus antigen and CCL5 expression by confocal microscopy using FITC-conjugated anti-HSV-1 Ab (green) and Alexa fluor 546-conjugated anti-CCL5 Ab (red). Similar results were found for WT (shown) and CCR5-/- mice (not shown). Note the lack of reactivity using isotypic control IgG. Uninfected mice showed an identical profile to the isotypic control-labeled tissue (data not shown). (b) Anti-CCL5 antibody (1 μg) conjugated with Alexa fluor 546 was pre-incubated with 500 pg of CCL5 or CCL2 for 30 minutes at 37° C. The antibodies were then added to prepared whole corneas from HSV-1-infected mice (24 hr post infection). Positive staining is denoted in red (indicated by yellow arrows). Note pre-incubation of anti-CCL5 antibody with CCL5 but not CCL2 completely blocks the recognition of antigen in the tissue showing specificity of the antibody. This figure is representative of two experiments for each time point for each group of mice evaluated. Magnification is 400x.
Figure 4
Reduction of Gr-1+ cell recruitment in CCR5-/- cornea following HSV-1 infection. C57BL/6 wild type and CCR5-/- mice were infected with 500 pfu/eye HSV-1. Mice were euthanized at the indicated time post infection (p.i.) and inspected for Gr-1+ cell infiltration and CCL5 expression by confocal microscopy using FITC-conjugated anti-Gr-1+ Ab (green) and Alexa fluor 546-conjugated anti-CCL5 Ab (red). Note the corneas from uninfected mice showed no detectable CCL5 expression or Gr-1+ cells. In separate experiments, Gr-1+ cells were found not to be Mac-3+. This figure is representative of two experiments for each time point for each group of mice evaluated. Magnification is 400x.
Figure 5
Aberrant T cell recruitment into the cornea of CCR5-/- following ocular HSV-1 infection. C57BL/6 wild type and CCR5-/- mice (n=5 mice/group) were infected with 500 pfu/eye HSV-1. Mice were euthanized 7 days post infection and inspected for CD3+ cell infiltration into the cornea using FITC-conjugated anti-CD3+ antibody (green) by confocal microscopy. Nuclear staining is represented by DAPI (blue). Magnification is 400x and represents a composite image of the cornea. Numbers represent the mean +/- SEM, n=10/group.
Figure 6
Increase in T cell infiltration in the nervous system of CCR5-/- mice following HSV-1 infection. C57BL/6 wild type (WT) and CCR5-/- mice (n=6-7 mice/group) were infected with 500 pfu/eye HSV-1. Mice were euthanized 7 days post infection and inspected for CD3+CD4+ and CD3+CD8+ T cell infiltration into the trigeminal ganglia (TG) and brain stem (BS) by flow cytometry. Upper panel is a representative result comparing CD8 T cell infiltration in the TG and BS of WT and CCR5-/- mice. Lower panel is a summary of the analysis. Bars represent mean +/- SEM, *p<.05 comparing WT to CCR5-/- groups.
Figure 7
Lack of response to recall antigen in CCR5-/- mice. C57BL/6 wild type and CCR5-/- mice were infected with 500 pfu/eye HSV-1 and euthanized 7 days post infection (p.i.). The cervical lymph nodes were removed and the cells were counted using Trypan blue. Panel A shows the total cell count for lymph nodes obtained from wild type and CCR5-/- mice (n = 15/group) and the percentage of CD4+ and CD8+ T cells within the lymph node population (n = 10/group). Bars represent mean +/- SEM. **p<.01 comparing the WT to CCR5-/- mice. The dotted line denotes the mean number of total leukocytes from uninfected CLN. Panel B shows the results of cytokine/chemokine production by cervical lymph node (CLN) or splenic cells (1.5 × 106 cells/well) obtained from mice 7 days p.i. and incubated with heat-inactivated HSV-1 (7.5 × 105 pfu) for 5 days. Supernatants were collected from the stimulated cells and assayed for cytokine/chemokine production using the BioPlex suspension array system or sandwich ELISA. Bars represent the mean +/- SEM for each cytokine/chemokine (n=5-10/group/analyte). *p<.05 comparing the wild type to CCR5-/- mice.
Figure 8
Cervical lymph node cells from CCR5-/- mice suppress HSV-1 infection in the brain stem in CCR5-/- recipient animals. One million cervical lymph node cells from day 6 HSV-1-infected C57BL/6 wild type (WT) or CCR5-/- mice (n=6/group) were inoculated intravenously into CCR5-/- mice infected 3 days previously with HSV-1 (1,000 pfu/eye). At day 7 post infection, the recipient animals were euthanized and the cornea, trigeminal ganglia (TG), and brain stem were removed, homogenized, and assayed for HSV-1 titers by plaque assay. Bars represent mean HSV-1 titer +/- SEM.
Similar articles
- Pathogenesis of herpetic stromal keratitis in CCR5- and/or CXCR3-deficient mice.
Komatsu K, Miyazaki D, Morohoshi K, Kuo CH, Kakimaru-Hasegawa A, Komatsu N, Namba S, Haino M, Matsushima K, Inoue Y. Komatsu K, et al. Curr Eye Res. 2008 Sep;33(9):736-49. doi: 10.1080/02713680802344716. Curr Eye Res. 2008. PMID: 18798077 - CXCL10/CXCR3-Dependent Mobilization of Herpes Simplex Virus-Specific CD8+ TEM and CD8+ TRM Cells within Infected Tissues Allows Efficient Protection against Recurrent Herpesvirus Infection and Disease.
Srivastava R, Khan AA, Chilukuri S, Syed SA, Tran TT, Furness J, Bahraoui E, BenMohamed L. Srivastava R, et al. J Virol. 2017 Jun 26;91(14):e00278-17. doi: 10.1128/JVI.00278-17. Print 2017 Jul 15. J Virol. 2017. PMID: 28468883 Free PMC article. - Herpes simplex virus type 1 induction of chemokine production is unrelated to viral load in the cornea but not in the nervous system.
Carr DJ, Campbell IL. Carr DJ, et al. Viral Immunol. 2006 Winter;19(4):741-6. doi: 10.1089/vim.2006.19.741. Viral Immunol. 2006. PMID: 17201669 Free PMC article. - CD4+ T cell migration into the cornea is reduced in CXCL9 deficient but not CXCL10 deficient mice following herpes simplex virus type 1 infection.
Wuest T, Farber J, Luster A, Carr DJ. Wuest T, et al. Cell Immunol. 2006 Oct;243(2):83-9. doi: 10.1016/j.cellimm.2007.01.001. Epub 2007 Feb 12. Cell Immunol. 2006. PMID: 17296171 Free PMC article. - [Battle with herpes for 37 years].
Shimomura Y. Shimomura Y. Nippon Ganka Gakkai Zasshi. 2015 Mar;119(3):145-66; discussion 167. Nippon Ganka Gakkai Zasshi. 2015. PMID: 25854108 Review. Japanese.
Cited by
- Insights into the pathogenesis of herpes simplex encephalitis from mouse models.
Mancini M, Vidal SM. Mancini M, et al. Mamm Genome. 2018 Aug;29(7-8):425-445. doi: 10.1007/s00335-018-9772-5. Epub 2018 Aug 23. Mamm Genome. 2018. PMID: 30167845 Free PMC article. Review. - Enhanced resistance of CXCR3 deficient mice to ocular HSV-1 infection is due to control of replication in the brain ependyma.
Kroll CM, Zheng M, Carr DJ. Kroll CM, et al. J Neuroimmunol. 2014 Nov 15;276(1-2):219-23. doi: 10.1016/j.jneuroim.2014.08.005. Epub 2014 Aug 8. J Neuroimmunol. 2014. PMID: 25139013 Free PMC article. - CCR5 deficiency drives enhanced natural killer cell trafficking to and activation within the liver in murine T cell-mediated hepatitis.
Ajuebor MN, Wondimu Z, Hogaboam CM, Le T, Proudfoot AE, Swain MG. Ajuebor MN, et al. Am J Pathol. 2007 Jun;170(6):1975-88. doi: 10.2353/ajpath.2007.060690. Am J Pathol. 2007. PMID: 17525265 Free PMC article. - CCR5 and Biological Complexity: The Need for Data Integration and Educational Materials to Address Genetic/Biological Reductionism at the Interface of Ethical, Legal, and Social Implications.
Bauss J, Morris M, Shankar R, Olivero R, Buck LN, Stenger CL, Hinds D, Mills J, Eby A, Zagorski JW, Smith C, Cline S, Hartog NL, Chen B, Huss J, Carcillo JA, Rajasekaran S, Bupp CP, Prokop JW. Bauss J, et al. Front Immunol. 2021 Dec 2;12:790041. doi: 10.3389/fimmu.2021.790041. eCollection 2021. Front Immunol. 2021. PMID: 34925370 Free PMC article. - An increase in herpes simplex virus type 1 in the anterior segment of the eye is linked to a deficiency in NK cell infiltration in mice deficient in CXCR3.
Carr DJ, Wuest T, Ash J. Carr DJ, et al. J Interferon Cytokine Res. 2008 Apr;28(4):245-51. doi: 10.1089/jir.2007.0110. J Interferon Cytokine Res. 2008. PMID: 18439102 Free PMC article.
References
- Algood HMS, Flynn JL. CCR5-deficient mice control Mycobacterium tuberculosis infection despite increased pulmonary lymphocytic infiltration. J. Immunol. 2004;173:3287–3296. - PubMed
- Appay V, Rowland-Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001;22:83–87. - PubMed
- Banerjee K, Biswas PS, Kim B, Lee S, Rouse BT. CXCR2-/- mice show enhanced susceptibility to herpetic stromal keratitis: A role for IL-6-induced neovascularization. J. Immunol. 2004;172:1237–1245. - PubMed
- Belnoue E, Kayibanda M, Deschemin JC, Viguier M, Mack M, Kuziel WA, Renia L. CCR5 deficiency decreases susceptibility to experimental cerebral malaria. Blood. 2003;101:4253–4259. - PubMed
- Biswas PS, Banerjee K, Kim B, Rouse BT. Mice transgenic for IL-1 receptor antagonist protein are resistant to herpetic stromal keratitis: Possible role for IL-1 in herpetic stromal keratitis pathogenesis. J. Immunol. 2004;172:3736–3744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS41249/NS/NINDS NIH HHS/United States
- P30 EY012190/EY/NEI NIH HHS/United States
- EY12190/EY/NEI NIH HHS/United States
- R01 NS041249/NS/NINDS NIH HHS/United States
- EY015566/EY/NEI NIH HHS/United States
- R21 EY015566/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials