Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex - PubMed (original) (raw)
Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex
Elyssa B Margolis et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Dopaminergic afferents arising from the ventral tegmental area (VTA) are crucial elements in the neural circuits that mediate arousal, motivation, and reinforcement. Two major targets of these afferents are the medial prefrontal cortex (mPFC) and the nucleus accumbens (NAc). Whereas dopamine (DA) in the mPFC has been implicated in working memory and attentional processes, DA in the NAc is required for responding to reward predictive cues. These distinct functions suggest a role for independent firing patterns of dopaminergic neurons projecting to these brain regions. In fact, DA release in mPFC and NAc can be differentially modulated. However, to date, electrophysiological studies have largely overlooked heterogeneity among VTA neurons. Here, we provide direct evidence for differential neurotransmitter control of DA neural activity and corresponding DA release based on projection target. Kappa opioid receptor agonists inhibit VTA DA neurons that project to the mPFC but not those that project to the NAc. Moreover, DA levels in the mPFC, but not the NAc, are reduced after local infusion of kappa opioid receptor agonists into the VTA. These findings demonstrate that DA release in specific brain regions can be independently regulated by opioid targeting of a subpopulation of VTA DA neurons. Selective control of VTA DA neurons projecting to the mPFC has important implications for understanding addiction, attention disorders, and schizophrenia, all of which are associated with DA dysfunction in the mPFC.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
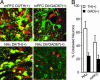
VTA projections to the mPFC and NAc arise from different populations of neurons. (A and B) Sample coronal brain sections showing injection tracks (Left) and tracer deposits (Right) resulting from the bilateral injection of the retrograde tracer DiI into the mPFC (A) or the NAc (B). (Scale bars: 2 mm.) (C1) Horizontal brain slice. The white box in the magnified midbrain region identifies the VTA region where experiments were completed (modified from ref. 36). (C2 and C3) Sample horizontal slices of midbrain region showing transported DiI (white) 7 days after mPFC (C2) or NAc (C3) injections. (Scale bars: 1 mm.) (D) VTA section from a rat injected with FluoroGold (green) into the mPFC and DiI (red) into the NAc shows a minority of colabeled (yellow) neurons. Neurons examined throughout the VTA show a relatively small amount of colabeling in this protocol (n = 3 rats).
Fig. 2.
Dopaminergic and GABAergic VTA neurons project to the mPFC and NAc. (A) VTA slices containing retrogradely labeled neurons (red) were immunohistologically processed for TH or GAD67 immunoreactivity (green), and colabeling (yellow) was observed. (B) Quantified colabeling across animals (NAc/TH, n = 3; NAc/GAD67, n = 4; mPFC/TH, n = 6; mPFC/GAD67, n = 7).
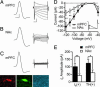
Fig. 3.
VTA neurons with a larger _I_h project to the mPFC. (A and B) Sample action potentials (calibration: 20 mV and 1 ms) and _I_h curves (calibration: 100 pA and 50 ms) measured in mPFC-projecting (A) and NAc-projecting (B) VTA neurons. (C) Sample mPFC-projecting neuron lacking an _I_h. This neuron was retrogradely labeled by DiI injection into the mPFC (red), filled with biocytin during recording (green), and was TH(−) (blue). (D) Among _I_h(+) neurons, the _I_h activated by a voltage step from −60 mV to −90, −100, −110, and −120 mV was significantly larger in mPFC projections (n = 18) than in NAc projections (n = 20). (Inset) _I_h was measured as the difference between the initial and final current during a 200-ms voltage step. (E) _I_h activation during a voltage step to −120 mV was significantly larger in confirmed TH(+), mPFC-projecting neurons (n = 10) than in NAc-projecting, TH(+) neurons (n = 7). ∗, P < 0.05; ∗∗, P < 0.01.
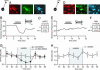
Fig. 4.
DA neurons that project to the mPFC but not to the NAc are inhibited by KOP-R agonists. (A) Example of a neuron labeled with DiI after injection into the mPFC (red), filled with biocytin during recording (green), and immunohistochemically confirmed as TH(+) (blue). (B) This neuron expressed a prominent _I_h (Inset; scale bar: 100 pA and 50 ms) and was inhibited by the KOP-R agonist U69593 (1 μM). (C) Summary of U69593 effects in mPFC-projecting _I_h(+) neurons. The numbers of confirmed TH(+) neurons are in parentheses. (D) Example neuron labeled with DiI after injection into the NAc (red), filled with biocytin during recording (green), and immunohistochemically confirmed as TH(+) (blue). (E) This neuron expressed an _I_h (Inset; calibration: 100 pA and 50 ms) and was unaffected by U69593 (1 μM). (F) U69593 inhibited only one NAc-projecting _I_h(+) neuron. The numbers of confirmed TH(+) neurons are in parentheses. (G) In vivo measurement of DA in the mPFC using microdialysis shows that perfusion of 5 μM U69593 into the VTA significantly decreased mPFC DA levels (n = 7). This effect was blocked by administration of the KOP-R-selective antagonist nor-BNI at 1 μM (n = 3). (H) Intra-VTA U69593 did not change DA levels in the NAc (n = 6).
Similar articles
- The Nucleus Reuniens of the Midline Thalamus Gates Prefrontal-Hippocampal Modulation of Ventral Tegmental Area Dopamine Neuron Activity.
Zimmerman EC, Grace AA. Zimmerman EC, et al. J Neurosci. 2016 Aug 24;36(34):8977-84. doi: 10.1523/JNEUROSCI.1402-16.2016. J Neurosci. 2016. PMID: 27559178 Free PMC article. - Role of dopamine projections from ventral tegmental area to nucleus accumbens and medial prefrontal cortex in reinforcement behaviors assessed using optogenetic manipulation.
Han X, Jing MY, Zhao TY, Wu N, Song R, Li J. Han X, et al. Metab Brain Dis. 2017 Oct;32(5):1491-1502. doi: 10.1007/s11011-017-0023-3. Epub 2017 May 19. Metab Brain Dis. 2017. PMID: 28523568 - Mesoaccumbal Dopamine Heterogeneity: What Do Dopamine Firing and Release Have to Do with It?
de Jong JW, Fraser KM, Lammel S. de Jong JW, et al. Annu Rev Neurosci. 2022 Jul 8;45:109-129. doi: 10.1146/annurev-neuro-110920-011929. Epub 2022 Feb 28. Annu Rev Neurosci. 2022. PMID: 35226827 Free PMC article. Review. - Functional relationship among medial prefrontal cortex, nucleus accumbens, and ventral tegmental area in locomotion and reward.
Tzschentke TM, Schmidt WJ. Tzschentke TM, et al. Crit Rev Neurobiol. 2000;14(2):131-42. Crit Rev Neurobiol. 2000. PMID: 11513242 Review.
Cited by
- Role of kappa-opioid receptors in stress and anxiety-related behavior.
Van't Veer A, Carlezon WA Jr. Van't Veer A, et al. Psychopharmacology (Berl). 2013 Oct;229(3):435-52. doi: 10.1007/s00213-013-3195-5. Epub 2013 Jul 9. Psychopharmacology (Berl). 2013. PMID: 23836029 Free PMC article. Review. - Prefrontal cortical kappa-opioid receptor modulation of local neurotransmission and conditioned place aversion.
Tejeda HA, Counotte DS, Oh E, Ramamoorthy S, Schultz-Kuszak KN, Bäckman CM, Chefer V, O'Donnell P, Shippenberg TS. Tejeda HA, et al. Neuropsychopharmacology. 2013 Aug;38(9):1770-9. doi: 10.1038/npp.2013.76. Epub 2013 Mar 29. Neuropsychopharmacology. 2013. PMID: 23542927 Free PMC article. - Distinct Neuromodulatory Effects of Endogenous Orexin and Dynorphin Corelease on Projection-Defined Ventral Tegmental Dopamine Neurons.
Mohammadkhani A, Qiao M, Borgland SL. Mohammadkhani A, et al. J Neurosci. 2024 Sep 25;44(39):e0682242024. doi: 10.1523/JNEUROSCI.0682-24.2024. J Neurosci. 2024. PMID: 39187377 Free PMC article. - Alcohol-induced plasticity in the dynorphin/kappa-opioid receptor system.
Sirohi S, Bakalkin G, Walker BM. Sirohi S, et al. Front Mol Neurosci. 2012 Sep 27;5:95. doi: 10.3389/fnmol.2012.00095. eCollection 2012. Front Mol Neurosci. 2012. PMID: 23060746 Free PMC article. - Kappa Opioid Receptors in Mesolimbic Terminals Mediate Escalation of Cocaine Consumption.
Gordon-Fennell L, Farero RD, Burgeno LM, Murray NL, Abraham AD, Soden ME, Stuber GD, Chavkin C, Zweifel LS, Phillips PEM. Gordon-Fennell L, et al. bioRxiv [Preprint]. 2023 Dec 23:2023.12.21.572842. doi: 10.1101/2023.12.21.572842. bioRxiv. 2023. PMID: 38187718 Free PMC article. Preprint.
References
- McClure S. M., Daw N. D., Montague P. R. Trends Neurosci. 2003;26:423–428. - PubMed
- Schultz W. Neuron. 2002;36:241–263. - PubMed
- Wise R. A. Nat. Rev. Neurosci. 2004;5:483–494. - PubMed
- Williams G. V., Goldman-Rakic P. S. Nature. 1995;376:572–575. - PubMed
- Chudasama Y., Robbins T. W. Psychopharmacology. 2004;174:86–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases