The chromatoid body of male germ cells: similarity with processing bodies and presence of Dicer and microRNA pathway components - PubMed (original) (raw)
The chromatoid body of male germ cells: similarity with processing bodies and presence of Dicer and microRNA pathway components
Noora Kotaja et al. Proc Natl Acad Sci U S A. 2006.
Abstract
The chromatoid body is a perinuclear, cytoplasmic cloud-like structure in male germ cells whose function has remained elusive. Here we show that the chromatoid body is related to the RNA-processing body of somatic cells. Dicer and components of microRNP complexes (including Ago proteins and microRNAs) are highly concentrated in chromatoid bodies. Furthermore, we show that Dicer interacts with a germ cell-specific chromatoid body component, the RNA helicase MVH (mouse VASA homolog). Thus, chromatoid bodies seem to operate as intracellular nerve centers of the microRNA pathway. Our findings underscore the importance of posttranscriptional gene regulation and of the microRNA pathway in the control of postmeiotic male germ cell differentiation.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
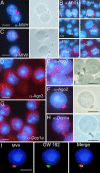
Localization of MVH and Ago proteins in chromatoid body. (A) Drying-down slides containing germ cells from stages II–V were labeled with anti-MVH antibody (red). The localization in chromatoid body was confirmed by parallel phase contrast microscopy. (B) Expression of MVH in chromatoid bodies during spermatogenesis. Squash preparations from stages I, V, VII, IX, and XII were stained with anti-MVH antibody (red) and studied by fluorescence microscopy. RS, round spermatid; PSc, pachytene spermatocyte; 2nd, secondary spermatocyte; m, meiotic division. (C) Localization of MIWI in chromatoid body. Germ cells from stages II–V were labeled with anti-MIWI antibody. (D and E) Ago3 is concentrated in chromatoid bodies. Germ cell squash preparations (D) at stage IV or drying-down slides containing germ cells from stages II–V (E) were immunolabeled by polyclonal anti-Ago3 antibody (red). (F) Ago2 was also shown to be concentrated in chromatoid bodies by using polyclonal anti-Ago2 antibody. (G and H) Localization of the P-body marker, the decapping enzyme Dcp1a, in chromatoid bodies. Germ cell squash preparations at stages III–IV (G) or drying-down slides from stages II–V (H) were immunostained by polyclonal anti-Dcp1a antibody. (I) Chromatoid body localization of a GW body marker protein. Patient sera against human GW182 protein (18033; a gift from Marvin J. Fritzler, University of Calgary, Calgary, Canada) were used for localization studies. For MVH detection, rabbit polyclonal anti-MVH antibody was used as a primary antibody. Alexa Fluor 594 anti-rabbit IgG or Alexa Fluor 488 anti-human IgG were used as secondary antibodies, and DAPI was used to stain nuclei (blue). (Scale bars: 5 μm.)
Fig. 2.
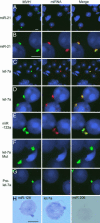
Localization of miRNA in chromatoid bodies. (A–G) In situ hybridization was performed with DIG-labeled locked nucleotide probes specific for different miRNAs. Except in A and C, samples fixed by the drying-down method were used for analysis. In A and C, squashed samples from stage II–VI seminiferous tubules were used. The miRNA-specific probes used in each panel are indicated. The prelet-7a probe was designed to detect the prelet-7a but not the mature let-7a. let-7a Mut is a mutant version of let-7a-specific probe. After in situ hybridization, miRNA signals were detected with the Roche fluorescent antibody enhancer set for DIG detection (red). MVH signals are in green, and the nucleus was stained with DAPI (blue). The merged pictures are also shown. (H) detection of miRNA by the colorimetric method. miR-206-specific probe is used as a negative control. (Scale bars: 5 μm.)
Fig. 3.
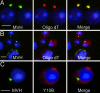
Poly(A)+ RNA is concentrated in chromatoid bodies. A Cy3-labeled oligo(dT) probe was used to detect mRNAs with a polyA tail in cells fixed by the drying-down method (A) and also in cells of stage V–VII squash preparations (B). After in situ hybridization with Cy3-labeled oligo(dT), MVH signals were revealed by immunofluorescence (green). DAPI was used to stain the nucleus. (C) Ribosomal RNA localization in round spermatids. Ribosomes localized to cytoplasmic regions (red) distinct from the chromatoid body positive for MVH (green). (Scale bars: 5 μm.)
Fig. 4.
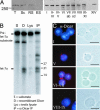
Localization of Dicer in chromatoid body. (A) Expression of Dicer protein in testis. Western blot analysis of the protein extracts of rat total testis cells (T), pachytene spermatocytes (Sc), round spermatids (RS), and elongated spermatids (ES), as well as of different stages of the rat seminiferous epithelial cycle (indicated as Roman numerals I–XIV), was performed by using anti-Dicer antibody. (B) premiRNA-processing activity in testis. prelet-7a substrate was processed when testis extract (Lys) or anti-Dicer immunoprecipitate (IP) was added to the reaction. Reaction with recombinant Dicer (D) was used as a positive control. (C) Dicer is expressed in the chromatoid body of late round spermatids. The drying-down slides were immunostained with the anti-Dicer 349 antibody and studied by both fluorescence microscopy (red, Dicer; blue, DNA) and phase contrast microscopy to confirm the chromatoid body localization. Similar staining was obtained with anti-Dicer 350 antibody. (Scale bars: 10 μm.)
Fig. 5.
Dicer interacts with MVH. (A) Interaction of the full-length EGFP-Dicer with MVH. IP was performed from COS-1 lysates overexpressing EGFP-Dicer and FLAG-MVH, FLAG-MVH(1–199), FLAG-MVH(1–496), or FLAG-MVHΔ284–503 with anti-FLAG antibody (α-FL), and the immunoblotting was performed by using either anti-Dicer antibody to detect coimmunoprecipitated Dicer or anti-FLAG antibody to detect MVH. (B) Schematic diagram of the MVH constructs used in this study. (C) The RNaseIII region of Dicer mediates the interaction with MVH. GST, GST-Helicase/PAZ containing the N-terminal helicase domain and the central PAZ domain of Dicer (H/PAZ), or GST-RNaseIII containing the C-terminal RNaseIII region and dsRNA binding domain of Dicer (RIII) was coexpressed with FLAG-MVH. After immunoprecipitation with anti-FLAG antibody, immunoblotting was done with anti-GST or anti-FLAG antibody. An asterisk indicates the IgG light chain. (D) Schematic representation of the Dicer mutants used in this study. (E) MVH and Dicer interact in vitro. Recombinant GST-MVH and Dicer-6xHis were incubated with glutathione Sepharose. After binding and washes, beads were run into a polyacrylamide gel, and immunoblotting was performed with anti-GST or anti-Dicer antibody.
Similar articles
- The chromatoid body of male germ cells: epigenetic control and miRNA pathway.
Nagamori I, Sassone-Corsi P. Nagamori I, et al. Cell Cycle. 2008 Nov 15;7(22):3503-8. doi: 10.4161/cc.7.22.6977. Epub 2008 Nov 12. Cell Cycle. 2008. PMID: 19001854 - Mouse RanBPM is a partner gene to a germline specific RNA helicase, mouse vasa homolog protein.
Shibata N, Tsunekawa N, Okamoto-Ito S, Akasu R, Tokumasu A, Noce T. Shibata N, et al. Mol Reprod Dev. 2004 Jan;67(1):1-7. doi: 10.1002/mrd.20009. Mol Reprod Dev. 2004. PMID: 14648869 - The chromatoid body: a germ-cell-specific RNA-processing centre.
Kotaja N, Sassone-Corsi P. Kotaja N, et al. Nat Rev Mol Cell Biol. 2007 Jan;8(1):85-90. doi: 10.1038/nrm2081. Nat Rev Mol Cell Biol. 2007. PMID: 17183363 Review. - Expression of vasa(vas)-related genes in germline cells and totipotent somatic stem cells of planarians.
Shibata N, Umesono Y, Orii H, Sakurai T, Watanabe K, Agata K. Shibata N, et al. Dev Biol. 1999 Feb 1;206(1):73-87. doi: 10.1006/dbio.1998.9130. Dev Biol. 1999. PMID: 9918696 - Chromatoid body and small RNAs in male germ cells.
Meikar O, Da Ros M, Korhonen H, Kotaja N. Meikar O, et al. Reproduction. 2011 Aug;142(2):195-209. doi: 10.1530/REP-11-0057. Epub 2011 Jun 7. Reproduction. 2011. PMID: 21652638 Review.
Cited by
- The mouse Balbiani body regulates primary oocyte quiescence via RNA storage.
Lei L, Ikami K, Diaz Miranda EA, Ko S, Wilson F, Abbott H, Pandoy R, Jin S. Lei L, et al. Commun Biol. 2024 Oct 2;7(1):1247. doi: 10.1038/s42003-024-06900-4. Commun Biol. 2024. PMID: 39358443 Free PMC article. - Ca(2+)/Calmodulin-Dependent Protein Kinase IV Promotes Interplay of Proteins in Chromatoid Body of Male Germ Cells.
Wang G, Zhang H, Wang L, Wang Y, Huang H, Sun F. Wang G, et al. Sci Rep. 2015 Jul 16;5:12126. doi: 10.1038/srep12126. Sci Rep. 2015. PMID: 26179157 Free PMC article. - Cytoplasmic compartmentalization of the fetal piRNA pathway in mice.
Aravin AA, van der Heijden GW, Castañeda J, Vagin VV, Hannon GJ, Bortvin A. Aravin AA, et al. PLoS Genet. 2009 Dec;5(12):e1000764. doi: 10.1371/journal.pgen.1000764. Epub 2009 Dec 11. PLoS Genet. 2009. PMID: 20011505 Free PMC article. - Phase transitioned nuclear Oskar promotes cell division of Drosophila primordial germ cells.
Kistler KE, Trcek T, Hurd TR, Chen R, Liang FX, Sall J, Kato M, Lehmann R. Kistler KE, et al. Elife. 2018 Sep 27;7:e37949. doi: 10.7554/eLife.37949. Elife. 2018. PMID: 30260314 Free PMC article. - Male germ cell apoptosis: regulation and biology.
Shaha C, Tripathi R, Mishra DP. Shaha C, et al. Philos Trans R Soc Lond B Biol Sci. 2010 May 27;365(1546):1501-15. doi: 10.1098/rstb.2009.0124. Philos Trans R Soc Lond B Biol Sci. 2010. PMID: 20403866 Free PMC article. Review.
References
- Sassone-Corsi P. Science. 2002;296:2176–2178. - PubMed
- Kimmins S., Sassone-Corsi P. Nature. 2005;434:583–589. - PubMed
- Steger K. Anat. Embryol. 2001;203:323–334. - PubMed
- Filipowicz W., Jaskiewicz L., Kolb F. A., Pillai R. S. Curr. Opin. Struct. Biol. 2005;15:331–341. - PubMed
- Zamore P. D., Haley B. Science. 2005;309:1519–1524. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous