Nicotinamide protects against ethanol-induced apoptotic neurodegeneration in the developing mouse brain - PubMed (original) (raw)
Nicotinamide protects against ethanol-induced apoptotic neurodegeneration in the developing mouse brain
Alessandro Ieraci et al. PLoS Med. 2006 Apr.
Abstract
Background: Exposure to alcohol during brain development may cause a neurological syndrome called fetal alcohol syndrome (FAS). Ethanol induces apoptotic neuronal death at specific developmental stages, particularly during the brain-growth spurt, which occurs from the beginning of third trimester of gestation and continues for several years after birth in humans, whilst occurring in the first two postnatal weeks in mice. Administration of a single dose of ethanol in 7-d postnatal (P7) mice triggers activation of caspase-3 and widespread apoptotic neuronal death in the forebrain, providing a possible explanation for the microencephaly observed in human FAS. The present study was aimed at determining whether nicotinamide may prevent ethanol-induced neurodegeneration.
Methods and findings: P7 mice were treated with a single dose of ethanol (5 g/kg), and nicotinamide was administered from 0 h to 8 h after ethanol exposure. The effects of nicotinamide on ethanol-induced activation of caspase-3 and release of cytochrome-c from the mitochondria were analyzed by Western blot (n = 4-7/group). Density of Fluoro-Jade B-positive cells and NeuN-positive cells was determined in the cingulated cortex, CA1 region of the hippocampus, and lateral dorsal nucleus of the thalamus (n = 5-6/group). Open field, plus maze, and fear conditioning tests were used to study the behavior in adult mice (n = 31-34/group). Nicotinamide reduced the activation of caspase-3 (85.14 +/- 4.1%) and the release of cytochrome-c (80.78 +/- 4.39%) in postnatal mouse forebrain, too. Nicotinamide prevented also the ethanol-induced increase of apoptosis. We demonstrated that ethanol-exposed mice showed impaired performance in the fear conditioning test and increased activity in the open field and in the plus maze. Administration of nicotinamide prevented all these behavioral abnormalities in ethanol-exposed mice.
Conclusions: Our findings indicate that nicotinamide can prevent some of the deleterious effects of ethanol on the developing mouse brain when given shortly after ethanol exposure. These results suggest that nicotinamide, which has been used in humans for the treatment of diabetes and bullous pemphigoid, may hold promise as a preventive therapy of FAS.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
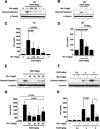
Figure 1. Nicotinamide Inhibits Ethanol-Induced Caspase-3 Activation
(A–D) P7 mice were injected with ethanol and received nicotinamide 2 h later. (A and B) Level of cleaved caspase-3 was analyzed by Western blot at 12 h (A) and 24 h (B) after ethanol administration with different doses of nicotinamide. (C and D) Densitometric quantification of cleaved caspase-3 at 12 h (ANOVA_F5,27_ = 10.989;p < 0.0001;n = 5–6 each treatment group) (C) and 24 h (ANOVA_F3,18_ = 8.851;p = 0.0008;n = 6 each treatment group) (D). (E and G) Nicotinamide was administered to P7 mice at different time points after ethanol exposure. Western blot analysis of caspase-3 activation 12 h after ethanol injection (E) and densitometric quantification (ANOVA_F5,33_ = 15.061;p < 0.0001;n = 6–7 each treatment group) (G). (F and H) Different doses of ethanol were administered to P7 mice and nicotinamide was injected 2 h later. Western blot analysis of caspase-3 activation 12 h after ethanol injection (F) and densitometric quantification (ANOVA_F6,28_ = 18.183;p < 0.0001;n = 5 each treatment group) (H). Values are shown as mean ± SD. Bonferroni correction for multiple comparisons revealed a significant difference between the ethanol treatment group and all other groups. ∗p < 0.0001.
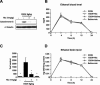
Figure 2. Nicotinamide Blocks Release of Cytochrome-C from Mitochondria
(A) Western blot analysis of cytochrome-c release in the cytosolic fraction 12 h after ethanol administration. (C) Densitometric quantification of cytochrome-c release (ANOVA_F3,20_ = 43.546_p <_ 0.0001;n = 6 each treatment group). Values are shown as mean ± SD. Bonferroni correction for multiple comparison revealed a significant difference between ethanol treatment group and all other groups. ∗p < 0.0001. (B and D) Nicotinamide (1mg/g) does not alter ethanol absorption or excretion. Ethanol levels in blood (B) and brain (D) were measured at different time points after 5g/kg of ethanol treatment in P7 mice. Nicotinamide was administered 2 h after ethanol exposure. No significant differences were seen between the different treatments (n = 4 each treatment group).
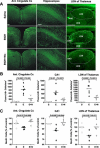
Figure 3. Nicotinamide Inhibits Ethanol-Induced Neurodegeneration
(A) Fluoro-Jade B staining 24 h after ethanol treatments. (B) Quantification of Fluoro-Jade–B positive cells in the cingulate cortex (ANOVA_F2,13_ = 25.541;p < 0.0001;n = 5–6 each treatment group), in the total CA1 region of hippocampus (ANOVA_F2,13_ = 9.988;p = 0.0024;n = 5–6 each treatment group), and in the LDN of thalamus (ANOVA_F2,13_ = 20.785;p < 0.0001;n = 5–6 each treatment group). (C) Total number of NeuN positive cells were counted 2 wk after ethanol administration in the cingulate cortex (ANOVA_F2,12_ = 9.894;p = 0.0029;n = 5 each treatment group), in the CA1 region of hippocampus (ANOVA_F2,12_ = 7.289;p = 0.0085;n = 5 each treatment group) and in the LDN of thalamus (ANOVA_F2,12_ = 5.551;p = 0.0196;n = 5 each treatment group). Values are shown as mean ± SD. Bonferroni correction for multiple comparison revealed a significant difference between ethanol treatment group and all other groups. S, saline; E, ethanol; Etoh+Nic, ethanol + nicotinamide. Scale bars 200μm.
Comment in
- Protection against prenatal alcohol-induced damage.
Spong CY. Spong CY. PLoS Med. 2006 Apr;3(4):e196. doi: 10.1371/journal.pmed.0030196. Epub 2006 Apr 18. PLoS Med. 2006. PMID: 16605309 Free PMC article. - Fetal alcohol syndrome and essential fatty acids.
Das UN. Das UN. PLoS Med. 2006 May;3(5):e247; author reply e248. doi: 10.1371/journal.pmed.0030247. Epub 2006 May 30. PLoS Med. 2006. PMID: 16719552 Free PMC article. No abstract available.
Similar articles
- Nicotinamide inhibits alkylating agent-induced apoptotic neurodegeneration in the developing rat brain.
Ullah N, Lee HY, Naseer MI, Ullah I, Suh JW, Kim MO. Ullah N, et al. PLoS One. 2011;6(12):e27093. doi: 10.1371/journal.pone.0027093. Epub 2011 Dec 2. PLoS One. 2011. PMID: 22164206 Free PMC article. Retracted. - Protective effect of pyruvate against ethanol-induced apoptotic neurodegeneration in the developing rat brain.
Ullah N, Naseer MI, Ullah I, Lee HY, Koh PO, Kim MO. Ullah N, et al. Neuropharmacology. 2011 Dec;61(8):1248-55. doi: 10.1016/j.neuropharm.2011.06.031. Epub 2011 Jul 23. Neuropharmacology. 2011. PMID: 21803053 - Drug-induced apoptotic neurodegeneration in the developing brain.
Olney JW, Wozniak DF, Jevtovic-Todorovic V, Farber NB, Bittigau P, Ikonomidou C. Olney JW, et al. Brain Pathol. 2002 Oct;12(4):488-98. doi: 10.1111/j.1750-3639.2002.tb00467.x. Brain Pathol. 2002. PMID: 12408236 Free PMC article. Review. - Glutamate signaling and the fetal alcohol syndrome.
Olney JW, Wozniak DF, Jevtovic-Todorovic V, Ikonomidou C. Olney JW, et al. Ment Retard Dev Disabil Res Rev. 2001;7(4):267-75. doi: 10.1002/mrdd.1037. Ment Retard Dev Disabil Res Rev. 2001. PMID: 11754521 Review.
Cited by
- Postnatal day 7 ethanol treatment causes persistent reductions in adult mouse brain volume and cortical neurons with sex specific effects on neurogenesis.
Coleman LG Jr, Oguz I, Lee J, Styner M, Crews FT. Coleman LG Jr, et al. Alcohol. 2012 Sep;46(6):603-12. doi: 10.1016/j.alcohol.2012.01.003. Epub 2012 May 7. Alcohol. 2012. PMID: 22572057 Free PMC article. - Nicotinamide Promotes Cell Survival and Differentiation as Kinase Inhibitor in Human Pluripotent Stem Cells.
Meng Y, Ren Z, Xu F, Zhou X, Song C, Wang VY, Liu W, Lu L, Thomson JA, Chen G. Meng Y, et al. Stem Cell Reports. 2018 Dec 11;11(6):1347-1356. doi: 10.1016/j.stemcr.2018.10.023. Epub 2018 Nov 29. Stem Cell Reports. 2018. PMID: 30503259 Free PMC article. - Fetal alcohol spectrum disorders and abnormal neuronal plasticity.
Medina AE. Medina AE. Neuroscientist. 2011 Jun;17(3):274-87. doi: 10.1177/1073858410383336. Epub 2011 Mar 7. Neuroscientist. 2011. PMID: 21383101 Free PMC article. Review. - Rehabilitation training using complex motor learning rescues deficits in eyeblink classical conditioning in female rats induced by binge-like neonatal alcohol exposure.
Wagner JL, Klintsova AY, Greenough WT, Goodlett CR. Wagner JL, et al. Alcohol Clin Exp Res. 2013 Sep;37(9):1561-70. doi: 10.1111/acer.12122. Epub 2013 May 3. Alcohol Clin Exp Res. 2013. PMID: 23647404 Free PMC article. - An Adrenalectomy Mouse Model Reflecting Clinical Features for Chronic Fatigue Syndrome.
Lee JS, Jeon YJ, Park SY, Son CG. Lee JS, et al. Biomolecules. 2020 Jan 1;10(1):71. doi: 10.3390/biom10010071. Biomolecules. 2020. PMID: 31906307 Free PMC article.
References
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. - PubMed
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. JAMA. 2003;290:2996–2999. - PubMed
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, et al. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. - PubMed
- Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: Mechanisms of damage and strategies for intervention. Exp Biol Med (Maywood) 2005;230:394–406. - PubMed
- Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: Increased brain damage with binge exposure. Alcohol Clin Exp Res. 1990;14:107–118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous