Function of the neuron-specific alternatively spliced isoforms of nonmuscle myosin II-B during mouse brain development - PubMed (original) (raw)
Function of the neuron-specific alternatively spliced isoforms of nonmuscle myosin II-B during mouse brain development
Xuefei Ma et al. Mol Biol Cell. 2006 May.
Abstract
We report that the alternatively spliced isoforms of nonmuscle myosin heavy chain II-B (NHMC II-B) play distinct roles during mouse brain development. The B1-inserted isoform of NMHC II-B, which contains an insert of 10 amino acids near the ATP-binding region (loop 1) of the myosin heavy chain, is involved in normal migration of facial neurons. In contrast, the B2-inserted isoform, which contains an insert of 21 amino acids near the actin-binding region (loop 2), is important for postnatal development of cerebellar Purkinje cells. Deletion of the B1 alternative exon, together with reduced expression of myosin II-B, results in abnormal migration and consequent protrusion of facial neurons into the fourth ventricle. This protrusion is associated with the development of hydrocephalus. Restoring the amount of myosin II-B expression to wild-type levels prevents these defects, showing the importance of total myosin activity in facial neuron migration. In contrast, deletion of the B2 alternative exon results in abnormal development of cerebellar Purkinje cells. Cells lacking the B2-inserted isoform show reduced numbers of dendritic spines and branches. Some of the B2-ablated Purkinje cells are misplaced in the cerebellar molecular layer. All of the B2-ablated mice demonstrated impaired motor coordination.
Figures
Figure 1.
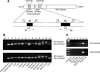
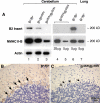
Expression of alternatively spliced isoforms of NMHC II-B in mice. (A) Diagram of NMHC II-B transcripts shows location of the alternative exons and primers used in RT-PCR analysis. (B) RT-PCR analysis of the B1- and B2-inserted NHMC II-B from tissues of adult mice using primers P1 and P2 for B1 insert (top) and P3 and P4 for the B2 insert (bottom). The B1 and B2 insert are only present in neuronal tissues. (C) RT-PCR analysis of the expression of the B1 and B2-inserted NMHC II-B in embryonic mouse brain (E12.5) using primers P5 and P2 for B1 insert (top) and P3 and P6 for B2 insert (bottom). Only the B1 insert is detected in embryonic brain and spinal cord. Adult mouse brain stem is included as a positive control, and adult mouse lung is used as a negative control.
Figure 2.
Generation of NMHC II-B alternative exon B1- or B2-ablated mice. (A) Ablation of exon B1. Diagrams of the wild-type allele, targeting construct and targeted allele for the generation of exon B1-ablated mice are shown. The HindII/EcoRI fragment containing the alternative B1 exon was replaced by a floxed Neor cassette. Bottom, Southern blot used for screening ES cell clones. Genomic DNA isolated from ES cell clones was digested with NcoI and probed with the indicated fragment. The wild-type allele generated a 12.7-kb fragment, whereas the targeted allele generated a 6.7-kb band. (B) Ablation of exon B2. The SpeI/StuI fragment containing the alternative B2 exon was replaced by a floxed Neor cassette. Bottom, Southern blot used for screening ES cell clones. Genomic DNA was digested by EcoRI and probed with the indicated fragment. The wild-type allele generated a 10-kb band, and the targeted allele generated an 8-kb band. Note that the constitutive exons are not shown.
Figure 3.
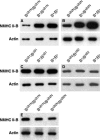
Immunoblot analyses of NMHC II-B expression of mouse brain extracts. (A) In the presence of the Neor cassette, the heterozygous (B+/BΔB1N) and homozygous (BΔB1N/BΔB1N) B1-ablated mice show a reduced amount of NMHC II-B expression compared with their wild-type littermates (B+/B+). (B) In the presence of Neor, the heterozygous (B+/BΔB2N) and homozygous (BΔB2N/BΔB2N) B2-ablated mice also show a reduced amount of NMHC II-B expression compared with their wild-type littermates (B+/B+). (C) After the removal of Neor from the targeted alleles, the heterozygous (B+/BΔB1) and homozygous (BΔB1/BΔB1) B1-ablated mice showed the same amount of NMHC II-B expression as their wild-type littermates (B+/B+). (D) The heterozygous (B+/BΔB2) and homozygous (BΔB2/BΔB2) B2-ablated mice also showed the same amount of NMHC II-B expression as their wild-type littermates (B+/B+) after the removal of Neor from the targeted alleles.(E) Littermates of BΔB1N/BΔB1N, BΔB2N/BΔB2N, and BΔB1N/BΔB2N generated by crossing BΔB1N/BΔB2N with BΔB1N/BΔB2N mice showed the same amount of NMHC II-B expression. NMHC II-B was detected using an antibody to the C-terminal sequence. Actin was used as a loading control in all these analyses.
Figure 4.
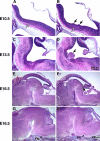
Impaired migration of facial neurons in BΔB1N/BΔB1N mice. (A and B) H&E staining of sagittal sections of embryonic mouse brains at E10.5 show that the migrating facial neurons in wild-type mice (B+/B+) form a well organized thin stream under the neuroepithlial cells parallel to the ventricular surface (A, white arrow). In BΔB1N/BΔB1N mice, the migrating facial neurons accumulate in one place (B, white arrow). Some of the BΔB1N/BΔB1N facial neurons are oriented perpendicularly to the ventricular surface and are beginning to protrude into the future fourth ventricle through the ependymal layer (B, black arrows). The most distal facial neurons, however, are still oriented normally as in wild-type mice. (C and D) Sagittal sections of embryonic brains near the midline at E13.5, a time when the facial neurons have almost completed their migration, show abnormal protrusion of facial neurons into the fourth ventricle in BΔB1N/BΔB1N mice (D, arrow) compared with wild-type (C). (E–H) Sagittal sections of E16.5 mouse brains in the middle (E and F) and lateral (G andH) region of the brain show protrusion of facial neurons (F, arrow) and reduced numbers of facial neurons (H, arrow) at their normal destination in BΔB1N/BΔB1N mice compared with the wild-type littermates (E and G). At least five mice from each genotype were analyzed.
Figure 5.
Development of hydrocephalus in BΔB1N/BΔB1N mice. (A and B) H&E staining of coronal sections of mouse brains at P21 shows that, as a result of severe hydrocephalus in the BΔB1N/BΔB1N mouse, the brain is massively disrupted (B) compared with the wild-type littermate (A). (C–F) Coronal sections of P21 mouse brains show the region around the aqueduct of Sylvius (C, AQ). In the wild-type mouse, the aqueduct of Sylvius is surrounded by an intact layer of ependymal cells (C and E). However, in BΔB1N/BΔB1N mice, commissural fibers run across the aqueduct (D, large arrow), disrupting the ependymal layer (D and F, small arrow), and the lumen of the aqueduct is completely blocked (F). (G–J) H&E staining of coronal sections of mouse brains shows enlarged lateral and third ventricles in BΔB1N/BΔB1N mice (panel H, LV and 3V) compared with wild-type littermates (G). Abnormal protrusion of the facial neurons into the fourth ventricle (4V) is observed in BΔB1N/BΔB1N mice (J, *), which is not seen in the wild-type littermate (I).
Figure 6.
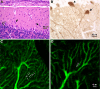
Abnormalities in the cerebellar Purkinje cell development in B2 insert-ablated mice. (A) H&E staining of a sagittal section of adult B2 insert-ablated mouse cerebellum shows misplacement of some of the cerebellar Purkinje cells (arrows) from the Purkinje cell layer (PL) to the molecular layer. (B) Immunohistochemical staining of a sagittal section of B2-ablated mouse cerebellum using antibodies against calbindin shows an abnormal orientation of the dendritic tree of some Purkinje cells (arrow) compared with their normal perpendicular orientation (arrowhead). (C and D) Immunofluorescence confocal images using antibodies against calbindin show a decreased number of dendritic branches and spines (arrows) in the Purkinje cells of a B2 insert-ablated mouse (D) compared with a wild-type littermate (C).
Figure 7.
Specificity of peptide antibodies against the B2-inserted NMHC II-B. (A) Immunoblot analysis of protein extracts prepared from the cerebellum and lung demonstrate that the B2 insert-specific antibody reacts only with B2-inserted NMHC II-B. Lanes 1, 2, and 3 are immunoblots of cerebellar extracts from wild-type, heterozygous and homozygous mice, demonstrating the specificity of the antibody for the B2 insert. Actin was used as a loading control. Lanes 4 and 5 show different loadings of the cerebellar extracts for B2-ablated mice and include an extract from lung tissue as a negative control (lane7). (B and C) Immunohistochemical staining of sagittal sections of mouse cerebellum demonstrate specific staining by the B2 antibody in wild-type cerebellar Purkinje cells (B, brown color, arrows), but no staining is detected in B2 insert-ablated mouse cerebellum (C, arrows).
Figure 8.
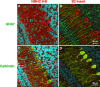
Immunofluorescence confocal images of mouse cerebellum at P10 using the indicated antibodies. (A) Costaining with antibodies for NMHC II-B (red) and glial-specific GFAP (green) shows that NMHC II-B is expressed in the Bergman glial fibers (yellow) as well as other cerebellar cells (red). (B) Costaining with antibodies specific for B2-inserted NMHC II-B (red) and GFAP (green) shows that the B2-inserted NMHC II-B does not costain with GFAP, indicating that B2-inserted NMHC II-B is not expressed in the Bergman glial cells and is confined to the Purkinje cells. (C) Costaining with antibodies for NMHC II-B (red) and the Purkinje cell-specific protein calbindin (green) shows that NMHC II-B and calbindin are both present in the Purkinje cells (yellow) but that NMHC-II-B is also present in other cells, too, such as granule cells (red). (D) Costaining with antibodies for B2-inserted NMHC II-B (red) and calbindin (green) shows that both proteins are confined to the Purkinje cells (yellow). Nuclei are stained with DAPI (blue).
Figure 9.
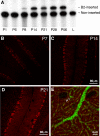
Temporal and regional expression of B2-inserted NMHC II-B in mouse cerebellum. (A) Autoradiogram after RT-PCR analysis of mRNA expression of B2-inserted NMHC II-B in wild-type mouse cerebellum during postnatal development. α-32P-dCTP was incorporated during PCR. Note that mRNA encoding the B2-inserted NMHC II-B is not detected in mouse cerebellum at P6, but it is expressed at P8, increases thereafter, and is maintained during adulthood. Adult lung (L) was used as a negative control for inserted NMHC II-B. (B–D) Immunofluorescence confocal microscopy of developing mouse cerebellum using B2 insert-specific antibody shows that B2-inserted NMHC II-B is detected in cerebellar Purkinje cells at P7 (B, red). Increased staining is seen at P14 and P21 (C and D, red). (E) A sagittal section of mouse cerebellum at P14 costained with B2 insert-specific antibody (green) and rhodamine-phalloidin for F-actin (red). Both the B2 insert-specific antibody and rhodamine-phalloidin showed punctuate staining. Yellow indicates colocalization of B2-inserted NMHC II-B and actin, indicating the dendritic spines (arrows).
Similar articles
- The B2 alternatively spliced isoform of nonmuscle myosin II-B lacks actin-activated MgATPase activity and in vitro motility.
Kim KY, Kawamoto S, Bao J, Sellers JR, Adelstein RS. Kim KY, et al. Biochem Biophys Res Commun. 2008 Apr 25;369(1):124-34. doi: 10.1016/j.bbrc.2007.11.108. Epub 2007 Dec 3. Biochem Biophys Res Commun. 2008. PMID: 18060863 Free PMC article. - Replacement of nonmuscle myosin II-B with II-A rescues brain but not cardiac defects in mice.
Bao J, Ma X, Liu C, Adelstein RS. Bao J, et al. J Biol Chem. 2007 Jul 27;282(30):22102-11. doi: 10.1074/jbc.M702731200. Epub 2007 May 22. J Biol Chem. 2007. PMID: 17519229 - Developmentally regulated expression of a nonmuscle myosin heavy chain IIB inserted isoform in rat brain.
Takahashi M, Hirano T, Uchida K, Yamagishi A. Takahashi M, et al. Biochem Biophys Res Commun. 1999 May 27;259(1):29-33. doi: 10.1006/bbrc.1999.0717. Biochem Biophys Res Commun. 1999. PMID: 10334910 - Distinct and redundant roles of the non-muscle myosin II isoforms and functional domains.
Wang A, Ma X, Conti MA, Adelstein RS. Wang A, et al. Biochem Soc Trans. 2011 Oct;39(5):1131-5. doi: 10.1042/BST0391131. Biochem Soc Trans. 2011. PMID: 21936777 Free PMC article. Review. - Characterization of isoform diversity in smooth muscle myosin heavy chains.
Kelley CA, Adelstein RS. Kelley CA, et al. Can J Physiol Pharmacol. 1994 Nov;72(11):1351-60. doi: 10.1139/y94-195. Can J Physiol Pharmacol. 1994. PMID: 7767878 Review.
Cited by
- Fox-3 and PSF interact to activate neural cell-specific alternative splicing.
Kim KK, Kim YC, Adelstein RS, Kawamoto S. Kim KK, et al. Nucleic Acids Res. 2011 Apr;39(8):3064-78. doi: 10.1093/nar/gkq1221. Epub 2010 Dec 21. Nucleic Acids Res. 2011. PMID: 21177649 Free PMC article. - Role of RNA binding proteins of the Drosophila behavior and human splicing (DBHS) family in health and cancer.
Takeiwa T, Ikeda K, Horie K, Inoue S. Takeiwa T, et al. RNA Biol. 2024 Jan;21(1):1-17. doi: 10.1080/15476286.2024.2332855. Epub 2024 Mar 29. RNA Biol. 2024. PMID: 38551131 Free PMC article. Review. - Structure, regulation, and mechanisms of nonmuscle myosin-2.
Chinthalapudi K, Heissler SM. Chinthalapudi K, et al. Cell Mol Life Sci. 2024 Jun 15;81(1):263. doi: 10.1007/s00018-024-05264-6. Cell Mol Life Sci. 2024. PMID: 38878079 Free PMC article. Review. - Systematic evaluation of isoform function in literature reports of alternative splicing.
Bhuiyan SA, Ly S, Phan M, Huntington B, Hogan E, Liu CC, Liu J, Pavlidis P. Bhuiyan SA, et al. BMC Genomics. 2018 Aug 28;19(1):637. doi: 10.1186/s12864-018-5013-2. BMC Genomics. 2018. PMID: 30153812 Free PMC article. - A human de novo mutation in MYH10 phenocopies the loss of function mutation in mice.
Tuzovic L, Yu L, Zeng W, Li X, Lu H, Lu HM, Gonzalez KD, Chung WK. Tuzovic L, et al. Rare Dis. 2013 Aug 14;1:e26144. doi: 10.4161/rdis.26144. eCollection 2013. Rare Dis. 2013. PMID: 25003005 Free PMC article.
References
- Altman, J., and Bayer, S. A. (1997). Development of the Cerebellar System in Relation to Its Evolution, Structure, and Functions, Boca Raton, FL: CRC Press.
- Babu, G. J., Loukianov, E., Loukianova, T., Pyne, G. J., Huke, S., Osol, G., Low, R. B., Paul, R. J., and Periasamy, M. (2001). Loss of SM-B myosin affects muscle shortening velocity and maximal force development. Nat. Cell Biol. 3, 1025–1029. - PubMed
- Bao, J., Jana, S. S., and Adelstein, R. S. (2005). Vertebrate nonmuscle myosin II isoforms rescue small interfering RNA-induced defects in COS-7 cell cytokinesis. J. Biol. Chem. 280, 19594–19599. - PubMed
- Chantler, P. D., and Wylie, S. R. (2003). Elucidation of the separate roles of myosins IIA and IIB during neurite outgrowth, adhesion and retraction. IEE Proc. Nanobiotechnol. 150, 111–125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases