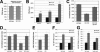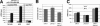Drosophila lacking microRNA miR-278 are defective in energy homeostasis - PubMed (original) (raw)
Comparative Study
. 2006 Feb 15;20(4):417-22.
doi: 10.1101/gad.374406.
Affiliations
- PMID: 16481470
- PMCID: PMC1369043
- DOI: 10.1101/gad.374406
Comparative Study
Drosophila lacking microRNA miR-278 are defective in energy homeostasis
Aurelio A Teleman et al. Genes Dev. 2006.
Erratum in
- Genes Dev. 2006 Apr 1;20(7):913. Maitra, Sushmita [added]
Abstract
The mechanisms that control energy homeostasis and tissue growth during development are closely linked through the insulin signal transduction pathway. Changes in the level of insulin and other hormones reflect the nutritional status of the animal to control circulating sugar levels and fat metabolism. Systemic defects in insulin responsiveness can lead to elevated circulating glucose levels and fat accumulation. Here we present evidence that the microRNA miR-278 plays a role in the control of energy homeostasis in Drosophila. miR-278 mutants have elevated insulin production and are correspondingly lean. Despite the elevated insulin levels, miR-278 mutants have elevated circulating sugar, mobilized from adipose-tissue glycogen stores. We provide evidence that miR-278 mutants are insulin resistant and that miR-278 acts through regulation of the expanded transcript.
Figures
Figure 1.
The miR-278 locus. (A) Schematic representation of the miR-278 locus. (Top) Overview of the genomic region (bar, 10 kb). P(EP)26043 and P(EP)EP2316 produced the tissue overgrowth phenotype shown in B. Both are ∼45 kb from the miR-278 sequence (blue arrowhead). (Bottom) Detail of the miR-278 region. miR-278 is located 300 bp from the 3′ end of the fus transcript. “knock-out” indicates the region replaced with the mini-white gene by homologous recombination; “Gal4 knock-in,” indicates the same region replaced by Gal4 and mini-white. (B) Adult wings from an engrailedGal4 control fly (green) and a fly expressing miR-278 in the posterior compartment under engrailedGal4 control from EP26043 (red). The wings are aligned in the anterior to show overgrowth of the posterior compartment expressing miR-278. (C) The predicted miR-278 hairpin, showing mutations identified as EMS-induced revertants of the overexpression phenotype. Blue shading indicates mature miR-278. (D) Adult wing expressing UAS-miR-278 in the posterior compartment under engrailedGal4 control. Note the more severe overgrowth and loss of the posterior cross-vein caused by UAS-miR-278. This resembles the phenotype of expanded mutant wings (not shown). (E) Northern blots showing miR-278 RNA. (Left) Overexpression in tubulinGal4 EP26043 flies compared with wild-type controls. (Right) Loss of miR-278 RNA in homozygous knock-out mutant flies (two independent isolates) compared with wild type. Thirty micrograms of total RNA per lane.
Figure 2.
miR-278 mutants are lean. (A) Average body weight of miR-278 mutant and control flies. Batches of 40 2-d-old males were assayed; experiment was done in quadruplicate. (B) Northern blot showing expression of miR-278 at different developmental stages. Loading control indicates the same filter probed to detect valine tRNA. (Bottom) Northern blot showing miR-278 expression in tissues dissected from third instar larvae. The amount of total RNA in each sample is indicted below. (C) Promoter GAL4 fusion driving GFP shows predominant fat body expression and weak body wall expression. (D) Total body triglycerides and total body protein were measured after controlled growth and aging. Plotted values are triglyceride levels normalized to protein levels. Batches of eight males were assayed; the experiment was done in triplicate. (E) Total body triglycerides normalized to total body protein for 8-d-old flies of the indicated genotypes. Batches of eight males were assayed; the experiment was done in triplicate. The low body fat level of the mutant was rescued by expression of UAS-miR-278 under control of miR-278-Gal4, which expresses Gal4 under control of the endogenous miR-278 regulatory elements.
Figure 3.
Elevated insulin and circulating sugar in miR-278 mutants. (A,B) Q-PCR on RNA from adult flies (A) or adult heads (B) normalized to rp49 RNA. Animals were grown under controlled conditions. AKH RNA was unaltered, whereas the Drosophila insulin-like peptides ILP2, ILP3, and ILP5 were upregulated. (C) Total body triglycerides normalized to total body protein for flies of the indicated genotypes. (D) Survival rates. Fifty first instar larvae were seeded per tube and eclosing adult flies counted. Samples indicate the average of 10 tubes. The reduced survival of the chico mutant was significantly rescued by reduction of miR-278 (_t_-test = 1.2 × 10–7). Error bars indicate standard deviation. (E) Ratio of glucose and trehalose levels in adult males grown under controlled conditions (Teleman et al. 2005b). Trehalase was omitted for the glucose measurement. (F) Expression level of 4E-BP in control and mutant tissues by Q-PCR. (G) Insulin-induced changes in gene expression in wild-type and mutant larvae by Q-PCR. Values show 4E-BP levels 1 h post-insulin injection as percentage of mock injection.
Figure 4
miR-278 regulates expanded. (A) Potential miR-278 target sites in the expanded 3′UTR. (B) Normalized luciferase activity for a reporter transgene containing the expanded 3′UTR compared with a control reporter with (black bars) and without (gray bars) coexpression of miR-278. Error bars indicate standard deviations. (C) Eyes from flies overexpressing expanded under sevenless-Gal4 control (left) and overexpressing expanded and miR-278 together (middle), and a control eye (right) for comparison. (D) Expanded protein in a wild-type wing imaginal disc visualized by antibody labeling (red). (E) Down-regulation of Expanded protein in the dorsal compartment of a wing disc expressing miR-278 under apterous-Gal4 control (green). The dorsal compartment has an extra fold due to tissue hyperplasia. (F) Expanded expression suppresses miR-278 overgrowth. Intervein L3–L4 area as percentage of total wing size for dpp-GAL4 driving indicated transgenes.
Figure 5
expanded regulation and the miR-278 mutant phenotype (A) Expanded mRNA levels and expanded locus transcription levels, as assayed by lacZ expression from an insertion, in control and mutant tissues, measured by Q-PCR. (B) Total body triglycerides normalized to total body protein for flies of the indicated genotypes. Batches of eight males were assayed. The experiment was done in triplicate. (C) ILP3 and ILP5 mRNA levels measured by Q-PCR in adult flies of the indicated genotypes.
Similar articles
- Drosophila miR-14 regulates insulin production and metabolism through its target, sugarbabe.
Varghese J, Lim SF, Cohen SM. Varghese J, et al. Genes Dev. 2010 Dec 15;24(24):2748-53. doi: 10.1101/gad.1995910. Genes Dev. 2010. PMID: 21159815 Free PMC article. - Coordination of insulin and Notch pathway activities by microRNA miR-305 mediates adaptive homeostasis in the intestinal stem cells of the Drosophila gut.
Foronda D, Weng R, Verma P, Chen YW, Cohen SM. Foronda D, et al. Genes Dev. 2014 Nov 1;28(21):2421-31. doi: 10.1101/gad.241588.114. Genes Dev. 2014. PMID: 25367037 Free PMC article. - Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B-null mice.
Choi YH, Park S, Hockman S, Zmuda-Trzebiatowska E, Svennelid F, Haluzik M, Gavrilova O, Ahmad F, Pepin L, Napolitano M, Taira M, Sundler F, Stenson Holst L, Degerman E, Manganiello VC. Choi YH, et al. J Clin Invest. 2006 Dec;116(12):3240-51. doi: 10.1172/JCI24867. J Clin Invest. 2006. PMID: 17143332 Free PMC article. - Peripheral signalling involved in energy homeostasis control.
Lancha A, Frühbeck G, Gómez-Ambrosi J. Lancha A, et al. Nutr Res Rev. 2012 Dec;25(2):223-48. doi: 10.1017/S0954422412000145. Nutr Res Rev. 2012. PMID: 23174510 Review. - Towards understanding regulation of energy homeostasis by ceramide synthases.
Bauer R. Bauer R. Results Probl Cell Differ. 2010;52:175-81. doi: 10.1007/978-3-642-14426-4_14. Results Probl Cell Differ. 2010. PMID: 20865380 Review.
Cited by
- Maternal loss of miRNAs leads to increased variance in primordial germ cell numbers in Drosophila melanogaster.
Kugler JM, Chen YW, Weng R, Cohen SM. Kugler JM, et al. G3 (Bethesda). 2013 Sep 4;3(9):1573-6. doi: 10.1534/g3.113.007591. G3 (Bethesda). 2013. PMID: 23893743 Free PMC article. - MicroRNA in carcinogenesis & cancer diagnostics: a new paradigm.
Ahmad J, Hasnain SE, Siddiqui MA, Ahamed M, Musarrat J, Al-Khedhairy AA. Ahmad J, et al. Indian J Med Res. 2013 Apr;137(4):680-94. Indian J Med Res. 2013. PMID: 23703335 Free PMC article. Review. - MicroRNAs as Regulators of Insulin Signaling: Research Updates and Potential Therapeutic Perspectives in Type 2 Diabetes.
Nigi L, Grieco GE, Ventriglia G, Brusco N, Mancarella F, Formichi C, Dotta F, Sebastiani G. Nigi L, et al. Int J Mol Sci. 2018 Nov 22;19(12):3705. doi: 10.3390/ijms19123705. Int J Mol Sci. 2018. PMID: 30469501 Free PMC article. Review. - Noncoding RNA Regulation of Hormonal and Metabolic Systems in the Fruit Fly Drosophila.
Chan KK, Chan TF, Bendena W, Hui JHL. Chan KK, et al. Metabolites. 2023 Jan 19;13(2):152. doi: 10.3390/metabo13020152. Metabolites. 2023. PMID: 36837772 Free PMC article. Review. - Resources and Methods for the Analysis of MicroRNA Function in Drosophila.
Mukherjee S, Sokol N. Mukherjee S, et al. Methods Mol Biol. 2022;2540:79-92. doi: 10.1007/978-1-0716-2541-5_3. Methods Mol Biol. 2022. PMID: 35980573 Review.
References
- Alvarez-Garcia I. and Miska, E.A. 2005. MicroRNA functions in animal development and human disease. Development 132: 4653–4662. - PubMed
- Arquier N., Bourouis, M., Colombani, J., and Leopold, P. 2005. Drosophila Lk6 kinase controls phosphorylation of eukaryotic translation initiation factor 4E and promotes normal growth and development. Curr. Biol. 15: 19–23. - PubMed
- Avruch J., Lin, Y., Long, X., Murthy, S., and Ortiz-Vega, S. 2005. Recent advances in the regulation of the TOR pathway by insulin and nutrients. Curr. Opin. Clin. Nutr. Metab. Care 8: 67–72. - PubMed
- Bartel D.P. 2004. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 116: 281–297. - PubMed
- Bentwich I., Avniel, A., Karov, Y., Aharonov, R., Gilad, S., Barad, O., Barzilai, A., Einat, P., Einav, U., Meiri, E., et al. 2005. Identification of hundreds of conserved and nonconserved human microRNAs. Nat. Genet. 37: 766–770. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases