Acetylation of Stat1 modulates NF-kappaB activity - PubMed (original) (raw)
Comparative Study
. 2006 Feb 15;20(4):473-85.
doi: 10.1101/gad.364306.
Affiliations
- PMID: 16481475
- PMCID: PMC1369049
- DOI: 10.1101/gad.364306
Comparative Study
Acetylation of Stat1 modulates NF-kappaB activity
Oliver H Krämer et al. Genes Dev. 2006.
Abstract
Acetylation of signaling molecules can lead to apoptosis or differentiation of carcinoma cells. The molecular mechanisms underlying these processes and the biological role of enzymes mediating the transfer or removal of an acetyl-group are currently under intense investigation. Our study shows that Stat1 is an acetylated protein. Stat1 acetylation depends on the balance between Stat1-associated histone deacetylases (HDACs) and histone acetyltransferases (HATs) such as CBP. Remarkably both inhibitors of HDACs and the cytokine interferon alpha alter this equilibrium and induce Stat1 acetylation. The analysis of Stat1 mutants reveals Lys 410 and Lys 413 as acetylation sites. Experiments with Stat1 mutants mimicking either constitutively acetylated or nonacetylated states show that only acetylated Stat1 is able to interact with NF-kappaB p65. As a consequence, p65 DNA binding, nuclear localization, and expression of anti-apoptotic NF-kappaB target genes decrease. These findings show how the acetylation of Stat1 regulates NF-kappaB activity and thus ultimately apoptosis.
Figures
Figure 1.
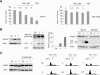
HDACi induce apoptosis in SK-37 cells. (A) The proliferation of SK-37 and NW-1539 melanoma cells was determined by MTT test after exposure to VPA (0.5–5 mM) or TSA (100 nM) for 48 h (SK-37) or 72 h (NW-1539); (0) untreated cells. (B) Induction of activated caspase 9 (Casp 9, activation denoted by an asterisk) and cleavage of full-length caspase 8 (Casp 8 fl) into the active subunits p43/41/18 were detected by Western blot after treatment of SK-37 cells with VPA (V, 1.5 mM) or TSA (T, 100 nM) for 48 h. Caspase 3 activity was measured by conversion of Ac-DEVD-pNA to pNA, which has an absorption peak at 405 nm. This increase is given relative to the activity of lysates from untreated cells (Ctl). HDACi-induced conversion of full-length caspase 3 (Casp 3 fl) to the active p17/19 subunits was analyzed in SK-37 and NW-450 cells. (C) Proteolytic cleavage of PARP and apoptotic chromatin fragmentation induced by VPA (1.5 mM) or TSA (100 nM) after 48 h were detected by Western blot and PI FACS analysis. Cotreatment of SK-37 cells with Z-VAD-FMK (Z, 100 μM) blocks HDACi-induced apoptosis.
Figure 2.
Correlation of Stat1 expression and apoptosis induction. (A) The time- and dose-dependent increase of Stat1 expression was investigated by Western blot. SK-37 cells were exposed to 1.5 mM VPA or 100 nM TSA for the indicated periods of time. Alternatively, cells were treated for 24 h with different concentrations of VPA (0.1–1.5 mM) or TSA (10–300 nM) as indicated or left untreated (0). (B) Expression of Stat1α in SK-37 and NW-450 melanoma cells treated with 1.5 mM VPA (V) or 100 nM TSA (T) for 24 h or left untreated (C) was analyzed by Western blot. (C) Expression of Stat1 and accumulation of hyperacetylated histone H4 (AcH4) in SK-37 and NW-1539 cells were analyzed after 24 h by Western blot. Cells were treated with VPA (V, 1.5 mM) or TSA (T, 30 nM) or left untreated (C). (D) Sensitivity of melanoma cell lines to VPA (V, 1.5 mM) and interferon α (α, 103 U/mL) was determined by MTT assay. Enhanced induction of apoptosis after treatment of SK-37 cells with VPA and interferon α (α/V) was detected by PI FACS analysis.
Figure 3.
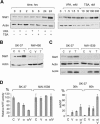
Stat1 sensitizes resistant melanoma cells to HDACi and interferon α. (A) Western blot analysis was employed to detect Stat1 expression and induction of apoptosis in NW-1539 cells transduced with SIEW (vector) or S-Stat1α-IEW (Stat1) and treated with VPA (1.5 mM) for 48 h. Asterisks denote activated forms of full-length caspase 3 or caspase 8. (B) DNA fragmentation was analyzed by PI FACS analysis after treatment with VPA (1.5 mM) or VPA and interferon α (103 U/mL) for 60 h. (Ctl) Untreated cells.
Figure 4.
Stat1 interferes with NF-κB function. (A) Expression of NF-κB target genes after HDAC inhibition and treatment with interferon α was investigated by Western blot analysis of SK-37 and NW-1539 cell lysates. Cells were incubated with 1.5 mM VPA (V), 30 nM TSA (T), or 103 U interferon α (IFN), or left untreated (C) for 48 h. (B) ABCD-assay with a biotinylated NF-κB consensus oligo was used to detect NF-κB–DNA binding under conditions described in A. (N) Nonrelevant biotinylated oligos. (C) NF-κB DNA binding was analyzed by EMSA of lysates from SK-37, NW-1539, and transduced NW-1539 cells (vector or Stat1) that were either untreated or treated with VPA (1.5 mM) for 48 h. Identity of the NF-κB–DNA complex was verified by p65 and p50 antibody supershifts (SS-AB). (D) NF-κB target gene expression was investigated in lysates of SK-37 cells transduced with an siRNA vector encoding scrambled RNA or an si-sequence against Stat1. Experimental conditions are as described in A. (E) NF-κB–DNA binding was investigated in lysates of SK-37 cells by ABCD-assay. Cells and conditions are as described in A. (F) NF-κB target gene expression was investigated in lysates of SK-37 cells transduced as stated in D and transfected with empty vector or pc3 HA-Stat1 containing mutations conferring resistance against the siRNA. Experimental conditions are as described in A. (G) NF-κB–DNA binding was investigated in lysates of SK-37 cells by ABCD-assay. Cells and conditions are as described in E.
Figure 5.
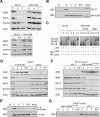
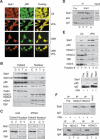
Complex formation and localization of Stat1α and NF-κB p65. (A) Interaction and colocalization of Stat1α and NF-κB p65 in SK-37 cells treated with VPA (1.5 mM, 24 h) and/or LMB (10 nM) were analyzed by immunofluorescence microscopy. (Ctl) Untreated. (B) Exclusion of p65 from the nuclear compartment after treatment of SK-37 cells with VPA (V, 1.5 mM), TSA (T, 100 nM), or interferon α (α, 103 U) for 24 h was confirmed by cellular fractionation and p65 Western blot. (C) Untreated. Reprobing was done with antibodies against Stat1. The affinity of the Stat1α antibody is not sufficient to detect nuclear Stat1. All other proteins detected serve as loading and fractionation controls. (C) U3A and 2fTGH cells were analyzed for cytoplasmic retention of p65 after incubation with 1.5 mM VPA (V) for 24 h by Western blot of cytosolic and nuclear fractions. (D) Interaction of Stat1α and NF-κB p65 in SK-37 cell lysates was investigated by Western blot of specific IPs as described in B (IP, Pre [preimmune serum], Input). (E) The composition of Stat1 complexes in SK-37 cells after treatment with VPA (1.5 mM, 48 h) was investigated by Western blot analysis of Superose 6 column fractions. (Lower panel) IP was used to verify the interaction of Stat1α with NF-κB. (F) HDAC1 and HDAC3 were precipitated from whole-cell extracts (IP). Western blot analysis was performed with an antibody against Stat1α/β. Treatment conditions are as described in D.
Figure 6.
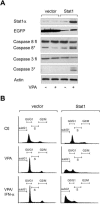
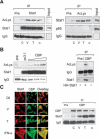
Acetylation of Stat1. (A) SK-37 cells were either treated with VPA (V, 1.5 mM), TSA (T, 30 nM), or interferon α (α, 103 U), or left untreated for 48 h. Endogenous Stat1α was immunoprecipitated from RIPA lysates and analyzed by Western blot with an antibody recognizing acetylated lysines (anti-AcLys, left). Reprobing of the same membrane confirms that the acetylation signal corresponds to Stat1α and shows efficacy and specificity of the IP. Anti-AcLys immunoprecipitates from RIPA lysates were probed with antibodies recognizing Stat1α/β or NF-κB p65. Preimmune serum was used as a control. Input lanes show 2% of the extract used for IP. (B) Increasing amounts of a CBP expression vector (1, 5, or 10 μg) were transfected into 293T cells. Stat1α was precipitated from RIPA lysates and probed with anti-AcLys. IPs with preimmune serum and IPs from cells transfected with the empty vector pc3.1 (10 μg) are controls. TNT-translated HA-Stat1 was acetylated in vitro as described (Gu and Roeder 1997) using immunoprecipitated CBP. (C) Interaction and colocalization of Stat1 and CBP in SK-37 cells were analyzed by immunofluorescence microscopy and IP of CBP. Cells were either treated with VPA (V, 1.5 mM), TSA (T, 30 nM), or interferon α (α, 103 U), or left untreated (C) for 48 h. (D) Acetylation levels of HA-Stat1ΔXbaI compared with full-length HA-Stat1α were determined by IP from 293T cell lysates as described in A. Cells were transfected with recombinant Stat1 and CBP vectors at a ratio of 5:1. (E) The experiment was performed as in D, except that HA-Stat1α or GFP-Stat1 410,413K → E were transfected. (F) NF-κB p65 was immunoprecipitated from 2fTGH or U3A cell extracts. The presence and acetylation of Stat1α were detected by Western blot as described in A. Cells were treated with 1.5 mM VPA for 24 h or left untreated. (G) Schematic representation of Stat1α showing positions of acetylated lysines and mutants generated. (NTD) N-terminal domain; (CC) coiled coil; (DBD) DNA-binding domain; (LD) linker domain; (TAD) transcriptional activation domain. Mutants are designated QQ (mutation of both K410 and K413 to Q) and RR (mutation of both K410 and K413 to R).
Figure 6.
Acetylation of Stat1. (A) SK-37 cells were either treated with VPA (V, 1.5 mM), TSA (T, 30 nM), or interferon α (α, 103 U), or left untreated for 48 h. Endogenous Stat1α was immunoprecipitated from RIPA lysates and analyzed by Western blot with an antibody recognizing acetylated lysines (anti-AcLys, left). Reprobing of the same membrane confirms that the acetylation signal corresponds to Stat1α and shows efficacy and specificity of the IP. Anti-AcLys immunoprecipitates from RIPA lysates were probed with antibodies recognizing Stat1α/β or NF-κB p65. Preimmune serum was used as a control. Input lanes show 2% of the extract used for IP. (B) Increasing amounts of a CBP expression vector (1, 5, or 10 μg) were transfected into 293T cells. Stat1α was precipitated from RIPA lysates and probed with anti-AcLys. IPs with preimmune serum and IPs from cells transfected with the empty vector pc3.1 (10 μg) are controls. TNT-translated HA-Stat1 was acetylated in vitro as described (Gu and Roeder 1997) using immunoprecipitated CBP. (C) Interaction and colocalization of Stat1 and CBP in SK-37 cells were analyzed by immunofluorescence microscopy and IP of CBP. Cells were either treated with VPA (V, 1.5 mM), TSA (T, 30 nM), or interferon α (α, 103 U), or left untreated (C) for 48 h. (D) Acetylation levels of HA-Stat1ΔXbaI compared with full-length HA-Stat1α were determined by IP from 293T cell lysates as described in A. Cells were transfected with recombinant Stat1 and CBP vectors at a ratio of 5:1. (E) The experiment was performed as in D, except that HA-Stat1α or GFP-Stat1 410,413K → E were transfected. (F) NF-κB p65 was immunoprecipitated from 2fTGH or U3A cell extracts. The presence and acetylation of Stat1α were detected by Western blot as described in A. Cells were treated with 1.5 mM VPA for 24 h or left untreated. (G) Schematic representation of Stat1α showing positions of acetylated lysines and mutants generated. (NTD) N-terminal domain; (CC) coiled coil; (DBD) DNA-binding domain; (LD) linker domain; (TAD) transcriptional activation domain. Mutants are designated QQ (mutation of both K410 and K413 to Q) and RR (mutation of both K410 and K413 to R).
Figure 7.
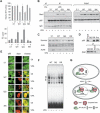
Identification of Stat1α acetylation as critical regulator of HDACi-induced apoptosis. (A) NW-1539 cells were transfected with Stat1α (WT, wild type), lysine mutants, or equal amounts of empty vector (pc3.1). Proliferation and apoptosis were scored 72 h later by MTT and PI FACS analysis, respectively. (WT) Wild type; (QQ) 410,413K → Q; (RR) 410,413K → R; (–) untreated; (V) 1.5 mM VPA. (B) Interaction of overexpressed wild-type (WT) and mutant Stat1α (QQ, RR) with NF-κB p65 in U3A cells was analyzed by IP and Western blot. Cells were incubated with 1.5 mM VPA for 48 h or left untreated. Input lanes are 2% of the lysate used for IP and are shown at expositons allowing signal comparison. (C) U3A cells were transfected and treated as described in B. Survivin expression was analyzed by Western blot. Detection of actin and AcH4 serve as loading and treatment controls, respectively. (D) SK-37 nuclear lysates were incubated with HA-Stat1 (QQ, 410,413K → Q; RR, 410,413K → R) immunoprecipitated in RIPA buffer or a precipitate formed with a control antibody (pre). Ten microliters of input and 20 μL of depleted nuclear extract were loaded (upper panel). The lower panel shows equal Stat1 IP efficiencies. (E) NF-κB p65 localization was analyzed by immunofluorescence microscopy of NW-1539 cells transfected and treated as described in B. Note: Compare transfected and nontransfected cells within each field. (F) DNA binding of NF-κB was investigated by EMSA with cell lysates of NW-1539 cells transfected and treated as described in B. (G) Model for acetylation-dependent Stat1–NF-κB cross-talk.
Similar articles
- Regulation of NF-kappaB action by reversible acetylation.
Greene WC, Chen LF. Greene WC, et al. Novartis Found Symp. 2004;259:208-17; discussion 218-25. Novartis Found Symp. 2004. PMID: 15171256 Review. - Histone deacetylase inhibitors block IFNγ-induced STAT1 phosphorylation.
Ginter T, Bier C, Knauer SK, Sughra K, Hildebrand D, Münz T, Liebe T, Heller R, Henke A, Stauber RH, Reichardt W, Schmid JA, Kubatzky KF, Heinzel T, Krämer OH. Ginter T, et al. Cell Signal. 2012 Jul;24(7):1453-60. doi: 10.1016/j.cellsig.2012.02.018. Epub 2012 Mar 7. Cell Signal. 2012. PMID: 22425562 - Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation.
Choi KC, Jung MG, Lee YH, Yoon JC, Kwon SH, Kang HB, Kim MJ, Cha JH, Kim YJ, Jun WJ, Lee JM, Yoon HG. Choi KC, et al. Cancer Res. 2009 Jan 15;69(2):583-92. doi: 10.1158/0008-5472.CAN-08-2442. Cancer Res. 2009. PMID: 19147572 - Histone deacetylase inhibition down-regulates cyclin D1 transcription by inhibiting nuclear factor-kappaB/p65 DNA binding.
Hu J, Colburn NH. Hu J, et al. Mol Cancer Res. 2005 Feb;3(2):100-9. doi: 10.1158/1541-7786.MCR-04-0070. Mol Cancer Res. 2005. PMID: 15755876 - Phosphorylation-acetylation switch in the regulation of STAT1 signaling.
Krämer OH, Heinzel T. Krämer OH, et al. Mol Cell Endocrinol. 2010 Feb 5;315(1-2):40-8. doi: 10.1016/j.mce.2009.10.007. Epub 2009 Oct 29. Mol Cell Endocrinol. 2010. PMID: 19879327 Review.
Cited by
- Molecular Effects of FDA-Approved Multiple Sclerosis Drugs on Glial Cells and Neurons of the Central Nervous System.
De Kleijn KMA, Martens GJM. De Kleijn KMA, et al. Int J Mol Sci. 2020 Jun 13;21(12):4229. doi: 10.3390/ijms21124229. Int J Mol Sci. 2020. PMID: 32545828 Free PMC article. Review. - The lysine deacetylase inhibitor Givinostat inhibits β-cell IL-1β induced IL-1β transcription and processing.
Dahllöf MS, Christensen DP, Lundh M, Dinarello CA, Mascagni P, Grunnet LG, Mandrup-Poulsen T. Dahllöf MS, et al. Islets. 2012 Nov-Dec;4(6):417-22. doi: 10.4161/isl.23541. Islets. 2012. PMID: 23486342 Free PMC article. - Regulation of STAT signaling by acetylation.
Zhuang S. Zhuang S. Cell Signal. 2013 Sep;25(9):1924-31. doi: 10.1016/j.cellsig.2013.05.007. Epub 2013 May 22. Cell Signal. 2013. PMID: 23707527 Free PMC article. Review. - Variation in transcription factor binding among humans.
Kasowski M, Grubert F, Heffelfinger C, Hariharan M, Asabere A, Waszak SM, Habegger L, Rozowsky J, Shi M, Urban AE, Hong MY, Karczewski KJ, Huber W, Weissman SM, Gerstein MB, Korbel JO, Snyder M. Kasowski M, et al. Science. 2010 Apr 9;328(5975):232-5. doi: 10.1126/science.1183621. Epub 2010 Mar 18. Science. 2010. PMID: 20299548 Free PMC article. - STAT1 signaling is not regulated by a phosphorylation-acetylation switch.
Antunes F, Marg A, Vinkemeier U. Antunes F, et al. Mol Cell Biol. 2011 Jul;31(14):3029-37. doi: 10.1128/MCB.05300-11. Epub 2011 May 16. Mol Cell Biol. 2011. PMID: 21576370 Free PMC article.
References
- Baumann S., Dostert, A., Novac, N., Bauer, A., Schmid, W., Fas, S.C., Krueger, A., Heinzel, T., Kirchhoff, S., Schütz, G., et al. 2005. Glucocorticoids inhibit activation-induced cell death (AICD) via direct DNA-dependent repression of the CD95 ligand gene by a glucocorticoid receptor dimer. Blood 106: 617–625. - PubMed
- Baus D. and Pfitzner, E. 2005. Specific function of STAT3, SOCS1 and SOCS3 in the regulation of proliferation and survival of classical Hodgkin lymphoma cells. Int. J. Can. (Epub ahead of print October 4, 2005. PMID: 16206268] - PubMed
- Blobel G.A. 2000. CREB-binding protein and p300: Molecular integrators of hematopoietic transcription. Blood 95: 745–755. - PubMed
- Chen L.F. and Greene, W.C. 2003. Regulation of distinct biological activities of the NF-κB transcription factor complex by acetylation. J. Mol. Med. 81: 549–557. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous