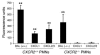Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung - PubMed (original) (raw)
. 2006 Mar;116(3):695-702.
doi: 10.1172/JCI27009. Epub 2006 Feb 16.
Affiliations
- PMID: 16485040
- PMCID: PMC1366502
- DOI: 10.1172/JCI27009
Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung
Jörg Reutershan et al. J Clin Invest. 2006 Mar.
Abstract
In models of acute lung injury, CXC chemokine receptor 2 (CXCR2) mediates migration of polymorphonuclear leukocytes (PMNs) into the lung. Since CXCR2 ligands, including CXCL1 and CXCL2/3, are chemotactic for PMNs, CXCR2 is thought to recruit PMNs by inducing chemotactic migration. In a model of PMN recruitment to the lung, aerosolized bacterial LPS inhalation induced PMN recruitment to the lung in wild-type mice, but not in littermate CXCR2-/- mice. Surprisingly, lethally irradiated wild-type mice reconstituted with CXCR2-/- BM still showed about 50% PMN recruitment into bronchoalveolar lavage fluid and into lung interstitium, but CXCR2-/- mice reconstituted with CXCR2-/- BM showed no PMN recruitment. Conversely, CXCR2-/- mice reconstituted with wild-type BM showed a surprisingly large defect in PMN recruitment, inconsistent with a role of CXCR2 on PMNs alone. Cell culture, immunohistochemistry, flow cytometry, and real-time RT-PCR were used to show expression of CXCR2 on pulmonary endothelial and bronchial epithelial cells. The LPS-induced increase in lung microvascular permeability as measured by Evans blue extravasation required CXCR2 on nonhematopoietic cells. Our data revealed what we believe to be a previously unrecognized role of endothelial and epithelial CXCR2 in LPS-induced PMN recruitment and lung injury.
Figures
Figure 1
LPS inhalation caused a significant recruitment of PMN into the BAL. In CXCR2 –/ – mice, PMN recruitment was completely abolished. In mice heterozygous for the CXCR2 allele (CXCR2+/ –), a reduction of ∼50% was observed. Data are mean ± SD of n = 6 mice in each group. Cytospins of LPS-exposed BAL in CXCR2+/+ and CXCR2 –/ – mice are shown above the corresponding bars. *P < 0.05 versus CXCR2+/+; #P < 0.05 versus no LPS.
Figure 2
Flow cytometry of lung homogenate. (A) PMNs were identified by their typical appearance in the forward scatter (FSC) and side scatter (SSC) and their expression of CD45 and CD11b (data not shown). GR-1 was injected intravenously to distinguish between intravascular and extravascular (i.e., interstitial after removal of BAL) PMNs. (B) In control animals (saline inhalation), 88% of PMNs were found intravascularly. (C) LPS caused transendothelial migration into the interstitium, as demonstrated by the appearance of CD11+GR-1– PMNs. (D) In contrast, transendothelial migration was suppressed in CXCR2 –/ – mice.
Figure 3
Chemotactic activity of LPS-exposed BAL from CXCR2+/+ mice and the 2 critical CXCR2 ligands, CXCL1 and CXCL2/3, was assessed in an in vitro transmigration assay. PMNs isolated from CXCR2+/+ mice showed significant migration toward LPS-exposed BAL, CXCL1, and CXCL2/3. PMNs from CXCR2 –/ – mice did not migrate toward the CXCR2 ligands, but still migrated toward LPS-exposed BAL from CXCR2 –/ – mice. Data are mean ± SD of 3 experiments. **P < 0.001 versus negative control (medium only).
Figure 4
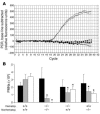
LPS-induced PMN migration into different lung compartments. (A) Complete reconstitution of hematopoietic cells after BMT was confirmed by RT-PCR (whole blood). Solid lines, positive (CXCR2+/+) and negative (CXCR2 –/ –) controls; lines with diamonds, CXCR2+/+ mice reconstituted with BM from CXCR2 –/ – mice. (B) When CXCR2+/+ mice were reconstituted with BM from CXCR2 –/ – mice, interstitial (gray bars) and BAL (white bars) PMN content was reduced by 40% and 50%, respectively. When CXCR2 –/ – mice were reconstituted with BM from CXCR2+/+ mice, the reduction was by 50% and 60%, respectively. LPS-induced accumulation of PMN in the pulmonary vasculature (black bars) did not differ among the groups. CXCR2+/+ mice reconstituted with BM from CXCR2+/+ and CXCR2 –/ – mice reconstituted with BM from CXCR2 –/ – served as positive and negative controls. Data are mean ± SD of n = 4 mice. *P < 0.05 versus positive control.
Figure 5
_CXCR2_mRNA expression in pulmonary endothelial cells (PECs) and BAL. Isolated pulmonary endothelial cells exhibited readily detectable amounts of CXCR2 mRNA. BAL from CXCR2+/+ also showed CXCR2 mRNA expression, but negligible CXCR2 mRNA was found in the BAL of CXCR2 –/ – mice. In CXCR2+/+ mice reconstituted with BM from CXCR2 –/ – mice, low levels of CXCR2 mRNA were detected, most likely derived from contamination with epithelial cells. CXCR2 mRNA expression was almost normal when CXCR2 –/ – mice were reconstituted with BM from CXCR2+/+ mice. CXCR2 mRNA levels were normalized to GAPDH and presented as mean ± SD of n = 3 samples.
Figure 6
CXCR2 protein expression shown by immunohistochemistry. (A) Lungs from CXCR2+/+ mice demonstrated CXCR2 expression among large vessels, small vessels (insert), and alveolar septal walls (arrow) and among the epithelial layer of large airways (C). No CXCR2 was detected in lungs from CXCR2 –/ – mice (B and D).
Figure 7
CXCR2 expression in lung homogenate. (B) Leukocytes (CD45+) and nonleukocytes (CD45–) were analyzed separately for their expression of CXCR2 (shown in C and A, respectively). (A) Among all CD45– cells, endothelial cells were identified by their expression of CD31. The majority of endothelial cells (∼67%) expressed CXCR2. In addition, CXCR2 was found in a large population of CD31– cells. (C) Among all leukocytes (CD45+), only PMNs (GR-1high) exhibited CXCR2 surface expression. Neither monocytes/macrophages (Mφs; GR-1intermediate) nor most lymphocytes (Lys; GR-1negative) showed detectable CXCR2 expression.
Figure 8
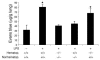
Pulmonary microvascular permeability was assessed in CXCR2+/+, CXCR2 –/ –, and chimeric mice. In CXCR2 –/ – mice and in CXCR2 –/ – mice reconstituted with BM from CXCR2+/+ mice, LPS-induced protein leakage was significantly reduced compared with CXCR2+/+ mice. In contrast, no protection was observed in CXCR2+/+ mice reconstituted with BM from CXCR2 –/ –. *P < 0.05 versus CXCR2+/+ mice. Data are mean ± SD of n = 4 mice.
Figure 9
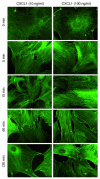
Endothelial cell response to CXCR2 activation was investigated by F-actin localization. Isolated pulmonary endothelial cells were treated with 10 or 100 ng/ml of recombinant CXCL1, and F-actin was localized by phalloidin staining. CXCL1 induced an increase in F-actin formation over time. Stress fibers appeared rapidly and were most pronounced at cell-cell borders. After 120 minutes, most endothelial cells appeared in a polygonal, retracted shape. Images are representative of 3 experiments with similar results.
Similar articles
- CXCR2 is critical to hyperoxia-induced lung injury.
Sue RD, Belperio JA, Burdick MD, Murray LA, Xue YY, Dy MC, Kwon JJ, Keane MP, Strieter RM. Sue RD, et al. J Immunol. 2004 Mar 15;172(6):3860-8. doi: 10.4049/jimmunol.172.6.3860. J Immunol. 2004. PMID: 15004193 - CXCR2 is essential for cerebral endothelial activation and leukocyte recruitment during neuroinflammation.
Wu F, Zhao Y, Jiao T, Shi D, Zhu X, Zhang M, Shi M, Zhou H. Wu F, et al. J Neuroinflammation. 2015 May 21;12:98. doi: 10.1186/s12974-015-0316-6. J Neuroinflammation. 2015. PMID: 25990934 Free PMC article. - Therapeutic effect of blocking CXCR2 on neutrophil recruitment and dextran sodium sulfate-induced colitis.
Farooq SM, Stillie R, Svensson M, Svanborg C, Strieter RM, Stadnyk AW. Farooq SM, et al. J Pharmacol Exp Ther. 2009 Apr;329(1):123-9. doi: 10.1124/jpet.108.145862. Epub 2009 Jan 8. J Pharmacol Exp Ther. 2009. PMID: 19131582 - Chemokine receptor CXCR2: physiology regulator and neuroinflammation controller?
Veenstra M, Ransohoff RM. Veenstra M, et al. J Neuroimmunol. 2012 May 15;246(1-2):1-9. doi: 10.1016/j.jneuroim.2012.02.016. Epub 2012 Mar 22. J Neuroimmunol. 2012. PMID: 22445294 Free PMC article. Review. - The role of CXCR2/CXCR2 ligands in acute lung injury.
Strieter RM, Keane MP, Burdick MD, Sakkour A, Murray LA, Belperio JA. Strieter RM, et al. Curr Drug Targets Inflamm Allergy. 2005 Jun;4(3):299-303. doi: 10.2174/1568010054022178. Curr Drug Targets Inflamm Allergy. 2005. PMID: 16101537 Review.
Cited by
- Alveolar epithelial cells mitigate neutrophilic inflammation in lung injury through regulating mitochondrial fatty acid oxidation.
Chung KP, Cheng CN, Chen YJ, Hsu CL, Huang YL, Hsieh MS, Kuo HC, Lin YT, Juan YH, Nakahira K, Chen YF, Liu WL, Ruan SY, Chien JY, Plataki M, Cloonan SM, Carmeliet P, Choi AMK, Kuo CH, Yu CJ. Chung KP, et al. Nat Commun. 2024 Aug 22;15(1):7241. doi: 10.1038/s41467-024-51683-1. Nat Commun. 2024. PMID: 39174557 Free PMC article. - A narrative review of chemokine receptors CXCR1 and CXCR2 and their role in acute respiratory distress syndrome.
Toya S, Struyf S, Huerta L, Morris P, Gavioli E, Minnella EM, Cesta MC, Allegretti M, Proost P. Toya S, et al. Eur Respir Rev. 2024 Jul 24;33(173):230172. doi: 10.1183/16000617.0172-2023. Print 2024 Jul. Eur Respir Rev. 2024. PMID: 39048127 Free PMC article. Review. - Activating A1 adenosine receptor signaling boosts early pulmonary neutrophil recruitment in aged mice in response to Streptococcus pneumoniae infection.
Simmons SR, Herring SE, Tchalla EYI, Lenhard AP, Bhalla M, Bou Ghanem EN. Simmons SR, et al. Immun Ageing. 2024 Jun 5;21(1):34. doi: 10.1186/s12979-024-00442-3. Immun Ageing. 2024. PMID: 38840213 Free PMC article. - Maximizing the Therapeutic Effect of Endothelin Receptor Antagonists in Pulmonary Fibrosis: A Paradigm for Treating the Disease.
Cantor J. Cantor J. Int J Mol Sci. 2024 Apr 10;25(8):4184. doi: 10.3390/ijms25084184. Int J Mol Sci. 2024. PMID: 38673771 Free PMC article. - Indole-3-carbinol attenuates lipopolysaccharide-induced acute respiratory distress syndrome through activation of AhR: role of CCR2+ monocyte activation and recruitment in the regulation of CXCR2+ neutrophils in the lungs.
Holloman BL, Wilson K, Cannon A, Nagarkatti M, Nagarkatti PS. Holloman BL, et al. Front Immunol. 2024 Mar 26;15:1330373. doi: 10.3389/fimmu.2024.1330373. eCollection 2024. Front Immunol. 2024. PMID: 38596679 Free PMC article.
References
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1334–1349. - PubMed
- Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L1137–L1145. - PubMed
- Azoulay E, et al. Deterioration of previous acute lung injury during neutropenia recovery. Crit. Care Med. 2002;30:781–786. - PubMed
- Reutershan J, Basit A, Galkina EV, Ley K. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L807–L815. - PubMed
- Doerschuk CM. Mechanisms of leukocyte sequestration in inflamed lungs. Microcirculation. 2001;8:71–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases