Adoptive transfer of nontransgenic mesenteric lymph node cells induces colitis in athymic HLA-B27 transgenic nude rats - PubMed (original) (raw)
Adoptive transfer of nontransgenic mesenteric lymph node cells induces colitis in athymic HLA-B27 transgenic nude rats
F Hoentjen et al. Clin Exp Immunol. 2006 Mar.
Abstract
HLA-B27 transgenic (TG) rats develop spontaneous colitis when colonized with intestinal bacteria, whereas athymic nude (rnu/rnu) HLA-B27 TG rats remain disease free. The present study was designed to determine whether or not HLA-B27 expression on T cells is required for development of colitis after transfer of mesenteric lymph node (MLN) cells into rnu/rnu HLA-B27 recipients. Athymic nontransgenic (non-TG) and HLA-B27 TG recipients received MLN cells from either TG or non-TG rnu/+ heterozygous donor rats that contain T cells. HLA-B27 TG rnu/rnu recipients receiving either non-TG or TG MLN cells developed severe colitis and had higher caecal MPO and IL-1beta levels, and their MLN cells produced more IFN-gamma and less IL-10 after in vitro stimulation with caecal bacterial lysate compared to rnu/rnu non-TG recipients that remained disease free after receiving either TG or non-TG cells. Interestingly, proliferating donor TG T cells were detectable one week after adoptive transfer into rnu/rnu TG recipients but not after transfer into non-TG recipients. T cells from either non-TG or TG donors induce colitis in rnu/rnu TG but not in non-TG rats, suggesting that activation of effector T cells by other cell types that express HLA-B27 is pivotal for the pathogenesis of colitis in this model.
Figures
Fig. 1
Caecal inflammation in recipients of MLN cell transfers. Representative sections of the caecum of rnu/rnu transgenic and nontransgenic recipient rats, eight weeks after transfer of MLN cells from rnu/+ transgenic or nontransgenic donors are shown at 10× magnification. Panels represent non-TG donor cells transferred into (a) non-TG or (c) TG recipients, and TG donor cells transferred into (b) non-TG or (d) TG recipients.
Fig. 2
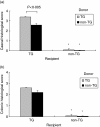
Caecal and colonic histological scores for recipients of MLN cell transfers. Intestinal tissue was collected from transgenic and nontransgenic rnu/rnu recipient rats, eight weeks after transfer of MLN cells from rnu/+ transgenic (□) or nontransgenic (▪) donors. Blinded histology scores for (a) caecal and (b) colonic (average of proximal, transverse, and distal colon) tissue are shown. Values represent mean ± SEM, n = 5–8 rats per group. *P < 0·005 for histological scores of non-TG recipient tissue compared to TG recipient tissue.
Fig. 3
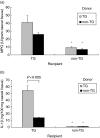
MPO and IL-1β in caecal tissue of cell transfer recipients. Transgenic and nontransgenic MLN cells from rnu/+ rats were transferred into rnu/rnu transgenic (□) or nontransgenic (▪) recipients. After eight weeks, caecal tissue was collected, homogenized, and (a) MPO and (b) IL-1β were determined in duplicate supernatants. Values represent mean ± SEM, n = 5–8 rats per group. *P < 0·005 for caecal tissue from non-TG recipients compared to TG recipients.
Fig. 4
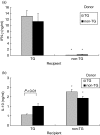
Cytokine production by recipient MLN cells. MLN cells were collected from transgenic and nontransgenic recipient rats, eight weeks after transfer of MLN cells from rnu/+ transgenic (□) or nontransgenic (▪) donors and cultured in the presence or absence of caecal bacterial lysate. After three days, supernatants were collected and (a) IFN-γ stimulated by caecal bacterial lysate at 50 µg/ml and (b) IL-10 stimulated by caecal bacterial lysate at 10 µg/ml were measured in triplicate supernatants by ELISA. Values represent mean ± SEM, n = 5–8 rats per group. IFN-γ levels were significantly lower (*P < 0·005) and IL-10 levels were significantly higher (*P < 0·005) in supernatants of caecal bacterial lysate stimulated MLN from non-TG recipients compared to TG recipients.
Fig. 5
IFN-γ production by caecal bacterial lysate stimulated MLN cells. MLN cells from rnu/+ TG rats were transferred into rnu/rnu non-TG or rnu/rnu TG recipients. After seven days, recipient MLN were harvested, and cells were stimulated with caecal bacterial lysate at 50 µg/ml or cultured without stimulation (medium). For comparison, MLN were also obtained from four month old SPF rnu/+ transgenic rats (not transplanted) and stimulated with caecal bacterial lysate or cultured with medium. Supernatants were collected after three days. IFN-γ was measured in triplicate supernatants by ELISA. Values represent mean ± SD of triplicate measurements from MLN cultures of individual animals. Results are representative of two independent experiments.
Fig. 6
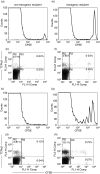
Equal numbers of CFSE labelled mesenteric lymph node cells from _rnu/_+ TG rats were transferred into rnu/rnu non-TG (a–d) or rnu/rnu TG recipients (e–h). After two (a,b,e,f) and seven days (c,d,g,h), recipient mesenteric lymph node cells were collected and analysed by flow cytometry to enumerate CFSE-labelled cells (histograms) and cells that express TCRαβ (dot plots). Values represent percent positive cells in the labelled quadrants. Results shown from one transgenic and one nontransgenic recipient evaluated at each time point are representative of two rats of each type evaluated in independent experiments for each time point.
References
- Sartor RB. Animal models of intestinal inflammation. In: Sartor RB, Sandborn W, editors. Kirsner's Inflammatory Bowel Diseases. Philadelphia: Elsevier; 2004. pp. 120–37.
- Hammer RE, Maika SD, Richardson JA, et al. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: an animal model of HLA-B27-associated human disorders. Cell. 1990;63:1099–112. - PubMed
- Haller D, Russo MP, Sartor RB, et al. IKK beta and phosphatidylinositol 3-kinase/Akt participate in non-pathogenic Gram-negative enteric bacteria-induced RelA phosphorylation and NF-kappa B activation in both primary and intestinal epithelial cell lines. J Biol Chem. 2002;277:38168–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK040249/DK/NIDDK NIH HHS/United States
- R01 DK 49249/DK/NIDDK NIH HHS/United States
- P30 DK034987/DK/NIDDK NIH HHS/United States
- P30 DK 34987/DK/NIDDK NIH HHS/United States
- K08 DK 02551/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous