Self-assembly of synthetic collagen triple helices - PubMed (original) (raw)
Self-assembly of synthetic collagen triple helices
Frank W Kotch et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Collagen is the most abundant protein in animals and the major component of connective tissues. Although collagen isolated from natural sources has long served as the basis for some biomaterials, natural collagen is difficult to modify and can engender pathogenic and immunological side effects. Collagen comprises a helix of three strands. Triple helices derived from synthetic peptides are much shorter (<10 nm) than natural collagen (approximately 300 nm), limiting their utility. Here, we describe the synthesis of short collagen fragments in which the three strands are held in a staggered array by disulfide bonds. Data from CD spectroscopy, dynamic light scattering, analytical ultracentrifugation, atomic force microscopy, and transmission electron microscopy indicate that these "sticky-ended" fragments self-assemble via intermolecular triple-helix formation. The resulting fibrils resemble natural collagen, and some are longer (>400 nm) than any known collagen. We anticipate that our self-assembly strategy can provide synthetic collagen-mimetic materials for a variety of applications.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
Structure and self-assembly of fragments 1 and 2. (A) Amino acid sequence of fragments 1 and 2. The glycine residue preceding the adjacent cysteines in the α2 strand establishes the requisite register for triple-helix formation with the identical α1 and α1′ strands. Hyp, (2_S_,4_R_)-4-hydroxyproline. (B) Representation of the self-assembly process; green, red, and blue are used simply to distinguish individual fragments.
Fig. 2.
Scheme for the synthesis of self-assembling fragments 1 and 2. tBu, _tert_-butyl; Acm, acetamidomethyl; Bu, butyl; TFE, 2,2,2-trifluoroethanol; iPr, isopropyl; HMPA, 4-hydroxymethylphenoxyacetyl; PEGA, polyethylene glycol acrylamide copolymer.
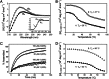
Fig. 3.
CD data for assemblies of fragments 1 and 2. (A) Spectra of assembled 1 and 2 (200 μM) at 4°C. (Inset) 1, 70 μM; 2, 100 μM. (B) Thermal denaturation of assembled 1 and 2 (200 μM), monitored at 226 nm. (C) Rate of assembly of fragments 1 and 2 (30 and 150 μM), over 90 min, monitored at 226 nm after a rapid transition from 90°C to 10°C. The [Θ]max for each sample was calculated as the difference between the ellipticity before denaturation (100% assembled) and the first data point at 10°C, thus omitting the initial jump in ellipticity (which was not resolvable) (47). The value of percent assembled was then calculated as the ratio of the observed recovery to [Θ]max. Data in A–C were from solutions in 50 mM HOAc(aq) at pH 2.9. (D) Thermal denaturation of assembled 1 and 2 (200 μM) in 67:33 MeOH/68 mM HOAc(aq) monitored at 226 nm.
Fig. 4.
AFM and TEM images for assemblies of fragments 1 and 2. (A) AFM height image of (1)n. (B) AFM height image of isolated (1)n (Upper) and section analysis of the data along the direction indicated by the arrow (Lower). (C) TEM image of (1)n after rotary shadowing with platinum. (Inset) An isolated fibril. (D) The same as A, except for (2)n. (E) The same as B, except for (2)n. (F) The same as C, except for (2)n.
Similar articles
- Peptide tessellation yields micrometre-scale collagen triple helices.
Tanrikulu IC, Forticaux A, Jin S, Raines RT. Tanrikulu IC, et al. Nat Chem. 2016 Nov;8(11):1008-1014. doi: 10.1038/nchem.2556. Epub 2016 Jul 11. Nat Chem. 2016. PMID: 27768103 Free PMC article. - Stabilization of short collagen-like triple helices by protein engineering.
Frank S, Kammerer RA, Mechling D, Schulthess T, Landwehr R, Bann J, Guo Y, Lustig A, Bächinger HP, Engel J. Frank S, et al. J Mol Biol. 2001 May 18;308(5):1081-9. doi: 10.1006/jmbi.2001.4644. J Mol Biol. 2001. PMID: 11352592 - The predominant roles of the sequence periodicity in the self-assembly of collagen-mimetic mini-fibrils.
Chen F, Strawn R, Xu Y. Chen F, et al. Protein Sci. 2019 Sep;28(9):1640-1651. doi: 10.1002/pro.3679. Protein Sci. 2019. PMID: 31299125 Free PMC article. - Targeting and mimicking collagens via triple helical peptide assembly.
Li Y, Yu SM. Li Y, et al. Curr Opin Chem Biol. 2013 Dec;17(6):968-75. doi: 10.1016/j.cbpa.2013.10.018. Epub 2013 Nov 5. Curr Opin Chem Biol. 2013. PMID: 24210894 Free PMC article. Review. - Pairwise interactions in collagen and the design of heterotrimeric helices.
Jalan AA, Hartgerink JD. Jalan AA, et al. Curr Opin Chem Biol. 2013 Dec;17(6):960-7. doi: 10.1016/j.cbpa.2013.10.019. Epub 2013 Nov 16. Curr Opin Chem Biol. 2013. PMID: 24252327 Review.
Cited by
- Shining light on collagen: expressing collagen in plants.
Brodsky B, Kaplan DL. Brodsky B, et al. Tissue Eng Part A. 2013 Jul;19(13-14):1499-501. doi: 10.1089/ten.TEA.2013.0137. Epub 2013 Apr 30. Tissue Eng Part A. 2013. PMID: 23521064 Free PMC article. - Fibrillar peptide gels in biotechnology and biomedicine.
Jung JP, Gasiorowski JZ, Collier JH. Jung JP, et al. Biopolymers. 2010;94(1):49-59. doi: 10.1002/bip.21326. Biopolymers. 2010. PMID: 20091870 Free PMC article. Review. - A Collagen-Mimetic Organic-Inorganic Hydrogel for Cartilage Engineering.
Valot L, Maumus M, Brunel L, Martinez J, Amblard M, Noël D, Mehdi A, Subra G. Valot L, et al. Gels. 2021 Jun 15;7(2):73. doi: 10.3390/gels7020073. Gels. 2021. PMID: 34203914 Free PMC article. - Supramolecular biomaterials.
Webber MJ, Appel EA, Meijer EW, Langer R. Webber MJ, et al. Nat Mater. 2016 Jan;15(1):13-26. doi: 10.1038/nmat4474. Nat Mater. 2016. PMID: 26681596 Review. - Supramolecular assembly of electrostatically stabilized, hydroxyproline-lacking collagen-mimetic peptides.
Krishna OD, Kiick KL. Krishna OD, et al. Biomacromolecules. 2009 Sep 14;10(9):2626-31. doi: 10.1021/bm900551c. Biomacromolecules. 2009. PMID: 19681603 Free PMC article.
References
- Ramshaw J. A. M., Werkmeister J. A., Glattauer V. Biotechnol. Genet. Eng. Rev. 1996;13:335–382. - PubMed
- Lee C. H., Singla A., Lee Y. Int. J. Pharm. 2001;221:1–22. - PubMed
- Cooperman L., Michaeli D. J. Am. Acad. Dermatol. 1984;10:638–646. - PubMed
- Sakaguchi M., Hori H., Hattori S., Irie S., Imai A., Yanagida M., Miyazawa H., Toda M., Inouye S. J. Allergy Clin. Immunol. 1999;104:695–699. - PubMed
- Lynn A. K., Yannas I. V., Bonfield W. J. Biomed. Mater. Res. B. 2004;71:343–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 AR050881/AR/NIAMS NIH HHS/United States
- 1RR13790/RR/NCRR NIH HHS/United States
- AR44276/AR/NIAMS NIH HHS/United States
- R01 AR044276/AR/NIAMS NIH HHS/United States
- AR50881/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases