Life cycle of connexins in health and disease - PubMed (original) (raw)
Review
Life cycle of connexins in health and disease
Dale W Laird. Biochem J. 2006.
Abstract
Evaluation of the human genome suggests that all members of the connexin family of gap-junction proteins have now been successfully identified. This large and diverse family of proteins facilitates a number of vital cellular functions coupled with their roles, which range from the intercellular propagation of electrical signals to the selective intercellular passage of small regulatory molecules. Importantly, the extent of gap-junctional intercellular communication is under the direct control of regulatory events associated with channel assembly and turnover, as the vast majority of connexins have remarkably short half-lives of only a few hours. Since most cell types express multiple members of the connexin family, compensatory mechanisms exist to salvage tissue function in cases when one connexin is mutated or lost. However, numerous studies of the last decade have revealed that mutations in connexin genes can also lead to severe and debilitating diseases. In many cases, single point mutations lead to dramatic effects on connexin trafficking, assembly and channel function. This review will assess the current understanding of wild-type and selected disease-linked mutant connexin transport through the secretory pathway, gap-junction assembly at the cell surface, internalization and degradation.
Figures
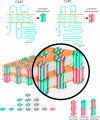
Figure 1. Schematic diagram illustrating the selective trans-junctional properties of gap junctions in intercellular communication
Gap-junction channels can be permeable to small molecules (A), small molecules with elongated shapes (B) or combinations of both molecular shapes (C). It is also important to note that the charge of the trans-junctional molecule also governs permeability characteristics. Gap junctions are typically not permeable to molecules exceeding 1 kDa (purple).
Figure 2. Assembly of connexins into gap junctions
Cx43 and Cx45, as examples of connexin family members, typically thread through the membrane four times, with the AT, CT and CL exposed to the cytoplasm. Connexin arrangement in the membrane also yields two extracellular loops designated EL-1 and EL-2. Six connexins oligomerize into a connexon or hemichannel that docks in homotypic, heterotypic and combined heterotypic/heteromeric arrangements. In total, as many as 14 different connexon arrangements can form when two members of the connexin family intermix.
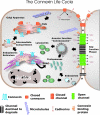
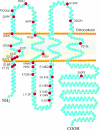
Figure 3. Life cycle of a connexin
Connexins typically co-translationally insert into the ER. If properly folded, it is expected that connexins are spared from ERAD, whereas in other cases they may be targeted for ERAD. For at least some members of the connexin family, complete oligomerization is delayed until the connexin passes through the intermediate compartment and reaches the distal elements of the Golgi apparatus, namely the TGN (_trans_-Golgi network). Pleiomorphic vesicles and transport intermediates are thought to deliver closed connexons to the cell surface, a process that is facilitated by microtubules. Connexons may function as hemichannels and exchange small molecules with the extracellular environment or laterally diffuse in a closed state to sites of cell–cell apposition and dock with connexons from an opposing cell. In conjunction with cadherin-based cell adhesion, gap-junction channels cluster into plaques, open and exchange secondary messengers. New gap-junction channels are recruited to the margins of gap-junction plaques and older channels are found in the centre of the plaques. Several connexin-binding proteins have been identified, and it is likely that one or more of these binding proteins regulate plaque formation and stability, possibly by acting as scaffolds to cytoskeletal elements. Gap-junction plaques and fragments of gap-junction plaques are internalized into one of two adjacent cells as a double-membrane structure commonly referred to as an annular junction, but renamed in the present review as connexosomes. Other pathways for connexin internalization may exist where connexons disassemble and enter the cell by classical endocytic pathways. Internalized gap junctions are targeted for degradation in lysosomes, although some evidence suggests a role in proteasomal degradation.
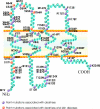
Figure 4. Cx43-binding proteins
Protein kinases known to phosphorylate Cx43 are shown along the top of a diagrammatically represented gap-junction plaque. A number of scaffolding proteins and proteins of unknown function that have been shown to bind directly or indirectly to Cx43 are shown along the bottom of the gap-junction plaque. It is important to note that it is not necessarily expected that all proteins shown here bind to Cx43 while it is a resident of the gap-junction plaque. MAP kinase, mitogen-activated protein kinase; CIP85, Cx43-interacting protein of 85 kDa.
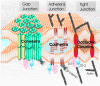
Figure 5. Junctional complexes arranged in a nexus
Gap junctions composed of connexins, adherens junctions consisting of cadherins and tight junctions made up of occludins and claudins are often closely arranged in epithelial cells and share common binding proteins that scaffold to actin and microfilaments. Binding-protein-mediated cross-talk allows these three junctional complexes to act as a nexus and be governed by some common regulatory events.
Figure 6. Deafness- and skin-disease-linked Cx26 mutations
Schematic diagram of Cx26 depicting a number of mutations associated with deafness (purple balls) and mutations associated with both deafness and skin diseases (orange balls).
Figure 7. ODDD-linked Cx43 mutations
Schematic diagram of Cx43 depicting the locations of 28 mutations (red balls) linked to ODDD.
Similar articles
- The gap junction proteome and its relationship to disease.
Laird DW. Laird DW. Trends Cell Biol. 2010 Feb;20(2):92-101. doi: 10.1016/j.tcb.2009.11.001. Epub 2009 Nov 26. Trends Cell Biol. 2010. PMID: 19944606 Review. - Gap junctions: structure and function (Review).
Evans WH, Martin PE. Evans WH, et al. Mol Membr Biol. 2002 Apr-Jun;19(2):121-36. doi: 10.1080/09687680210139839. Mol Membr Biol. 2002. PMID: 12126230 Review. - Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation.
Laird DW. Laird DW. Biochim Biophys Acta. 2005 Jun 10;1711(2):172-82. doi: 10.1016/j.bbamem.2004.09.009. Biochim Biophys Acta. 2005. PMID: 15955302 Review. - Regulation of gap junction intercellular communication by the ubiquitin system.
Kjenseth A, Fykerud T, Rivedal E, Leithe E. Kjenseth A, et al. Cell Signal. 2010 Sep;22(9):1267-73. doi: 10.1016/j.cellsig.2010.03.005. Epub 2010 Mar 4. Cell Signal. 2010. PMID: 20206687 Review. - Connexins and their channels in cell growth and cell death.
Vinken M, Vanhaecke T, Papeleu P, Snykers S, Henkens T, Rogiers V. Vinken M, et al. Cell Signal. 2006 May;18(5):592-600. doi: 10.1016/j.cellsig.2005.08.012. Epub 2005 Sep 23. Cell Signal. 2006. PMID: 16183253 Review.
Cited by
- Altered expression of connexin43 and phosphorylation connexin43 in glioma tumors.
Ye XY, Jiang QH, Hong T, Zhang ZY, Yang RJ, Huang JQ, Hu K, Peng YP. Ye XY, et al. Int J Clin Exp Pathol. 2015 May 1;8(5):4296-306. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26191122 Free PMC article. - Changes in connexin43 expression and localization during pancreatic cancer progression.
Solan JL, Hingorani SR, Lampe PD. Solan JL, et al. J Membr Biol. 2012 Jun;245(5-6):255-62. doi: 10.1007/s00232-012-9446-2. Epub 2012 Jun 23. J Membr Biol. 2012. PMID: 22729649 Free PMC article. - Connexin-Containing Vesicles for Drug Delivery.
Hanafy MS, Cui Z. Hanafy MS, et al. AAPS J. 2024 Jan 24;26(1):20. doi: 10.1208/s12248-024-00889-8. AAPS J. 2024. PMID: 38267725 Review. - Marine sponge depsipeptide increases gap junction length in HTC cells transfected with Cx43-GFP.
Rangel M, Ionta M, Cristina Pfister S, Adolpho Sant'anna Ferreira R, Maria Machado-Santelli G. Rangel M, et al. Cell Biol Int Rep (2010). 2010;17(1):e00003. doi: 10.1042/CBR20100003. Epub 2010 Aug 11. Cell Biol Int Rep (2010). 2010. PMID: 23119141 Free PMC article. - Degradation of gap junction connexins is regulated by the interaction with Cx43-interacting protein of 75 kDa (CIP75).
Kopanic JL, Schlingmann B, Koval M, Lau AF, Sorgen PL, Su VF. Kopanic JL, et al. Biochem J. 2015 Mar 15;466(3):571-85. doi: 10.1042/BJ20141042. Biochem J. 2015. PMID: 25583071 Free PMC article.
References
- Alexander D. B., Goldberg G. S. Transfer of biologically important molecules between cells through gap junction channels. Curr. Med. Chem. 2003;10:2045–2058. - PubMed
- Goodenough D. A., Goliger J. A., Paul D. L. Connexins, connexons, and intercellular communication. Annu. Rev. Biochem. 1996;65:475–502. - PubMed
- Saez J. C., Berthoud V. M., Branes M. C., Martinez A. D., Beyer E. C. Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 2003;83:1359–1400. - PubMed
- Sohl G., Willecke K. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004;62:228–232. - PubMed
- Sohl G., Willecke K. An update on connexin genes and their nomenclature in mouse and man. Cell Commun. Adhes. 2003;10:173–180. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous