Evolution of the mammalian placenta revealed by phylogenetic analysis - PubMed (original) (raw)
Evolution of the mammalian placenta revealed by phylogenetic analysis
Derek E Wildman et al. Proc Natl Acad Sci U S A. 2006.
Abstract
The placenta is essential for the success of therian mammalian reproduction. Intense selective pressure has shaped changes in placental anatomy and function during mammalian cladogenesis. Here we challenge the view that the hemochorial placenta is a derived feature in haplorhine primates. Using phylogenetic and statistical analyses of molecular and morphological data, we demonstrate that the ancestral eutherian mammalian placenta had the distinctive features of (i) hemochorial placental interface, (ii) a discoid shape, and (iii) a labyrinthine maternofetal interdigitation. These results reveal that the first eutherians had a deeply invasive placenta and imply that the major role of the placenta in sustaining pregnancy and promoting gestational development existed throughout the eutherian lineage that descended to humans from the last common ancestor of placental mammals. The ancestral state reconstructions demonstrate both clade-specific patterns of placentation and specific cases of convergent evolution within individual eutherian clades. Determining the mammalian pattern of change in placental morphology is important for understanding the evolutionary pressures faced by these lineages. The effects of selection pressures on the efficiency of placentation may stem from changes in nutritional demand, gestational length, number of embryos per pregnancy, uterine shape, and maternal body constitution. The influence of these factors on placental development needs further investigation.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
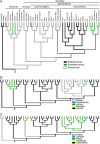
Fig. 1.
Phylogenetic relationships among major placental mammalian groups. Four major superordinal placental (i.e., eutherian) mammalian clades are supported by molecular data (9, 13). These clades are the Afrotheria, Xenartha, Euarchontaglires, and Laurasiatheria. All studies also support the grouping of Euarchontaglires and Laurasiatheria as sister taxa in a larger clade (Boreoeutheria). The phylogenetic branching order at the root of the tree is controversial (–13). (A) Depiction of the Afrotheria as sister to the remaining three clades (i.e., Notolegia). (B) Depiction of the Xenartha as sister to the remaining three clades. A third choice in which Boreoeutheria is sister to a clade that consists of Xenartha and Afrotheria is not supported by parsimony analysis (Table 1).
Fig. 2.
The evolution of morphological placental features in mammals. Parsimony reconstructions of internal nodes are shown for three morphological features (see Materials and Methods for details of reconstruction methodology). Taxon names and branching order are identical in all panels of the figure. Only the Afrotheria + Notolegia tree (i.e., 9) is shown. The data for all tree topologies examined is available as supporting information. (A) The placental interface describes the degree of invasiveness of fetal (i.e., placental) tissue into maternal tissue, with epitheliochorial being least invasive and hemochorial being most invasive. (B) The shape of the contact zone between fetal and uterine tissues. (C) The form of interdigitation between fetal and maternal tissues. Parsimony and Markov model likelihood reconstructions were constructed by using the data file available as supporting information.
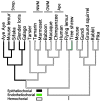
Fig. 3.
The evolution of the placental interface in primates. Parsimony reconstructions of internal nodes are shown for the placental interface character (see Materials and Methods for details of reconstruction methodology). Parsimony and Markov model likelihood reconstructions were constructed by using the data file available as supporting information. Strep., Strepsirrhine; NWM, New World monkeys; OWM, Old World monkeys.
Similar articles
- The current molecular phylogeny of Eutherian mammals challenges previous interpretations of placental evolution.
Vogel P. Vogel P. Placenta. 2005 Sep-Oct;26(8-9):591-6. doi: 10.1016/j.placenta.2004.11.005. Epub 2005 Jan 27. Placenta. 2005. PMID: 16085037 Review. - Evolution of the placenta in eutherian mammals.
Carter AM, Mess A. Carter AM, et al. Placenta. 2007 Apr;28(4):259-62. doi: 10.1016/j.placenta.2006.04.010. Epub 2006 Jun 15. Placenta. 2007. PMID: 16780944 Review. - Genomics, the diversification of mammals, and the evolution of placentation.
Carter AM. Carter AM. Dev Biol. 2024 Dec;516:167-182. doi: 10.1016/j.ydbio.2024.08.011. Epub 2024 Aug 20. Dev Biol. 2024. PMID: 39173812 Review. - A review of inter- and intraspecific variation in the eutherian placenta.
Gundling WE Jr, Wildman DE. Gundling WE Jr, et al. Philos Trans R Soc Lond B Biol Sci. 2015 Mar 5;370(1663):20140072. doi: 10.1098/rstb.2014.0072. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 25602076 Free PMC article. Review. - Placentation and maternal investment in mammals.
Capellini I, Venditti C, Barton RA. Capellini I, et al. Am Nat. 2011 Jan;177(1):86-98. doi: 10.1086/657435. Epub 2010 Nov 18. Am Nat. 2011. PMID: 21087154
Cited by
- Scar matrix drives Piezo1 mediated stromal inflammation leading to placenta accreta spectrum.
Wenqiang D, Novin A, Liu Y, Afzal J, Suhail Y, Liu S, Gavin NR, Jorgensen JR, Morosky CM, Figueroa R, Schmidt TA, Sanders M, Brewer MA, Kshitiz. Wenqiang D, et al. Nat Commun. 2024 Sep 27;15(1):8379. doi: 10.1038/s41467-024-52351-0. Nat Commun. 2024. PMID: 39333481 Free PMC article. - Convergently evolved placental villi show multiscale structural adaptations to differential placental invasiveness.
Laundon D, Sengers BG, Thompson J, Harris SE, Beasley O, Basford PJ, Katsamenis OL, Goggin P, Derisoud E, Fanelli D, Bocci C, Camillo F, Shotton J, Constable-Dakeyne G, Gostling NJ, Chavatte-Palmer P, Lewis RM. Laundon D, et al. Biol Lett. 2024 Mar;20(3):20240016. doi: 10.1098/rsbl.2024.0016. Epub 2024 Mar 27. Biol Lett. 2024. PMID: 38531417 Free PMC article. - Roles of retrovirus-derived PEG10 and PEG11/RTL1 in mammalian development and evolution and their involvement in human disease.
Shiura H, Kitazawa M, Ishino F, Kaneko-Ishino T. Shiura H, et al. Front Cell Dev Biol. 2023 Sep 29;11:1273638. doi: 10.3389/fcell.2023.1273638. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37842090 Free PMC article. Review. - The effect of placentation type, litter size, lactation and gestation length on cancer risk in mammals.
Dujon AM, Vincze O, Lemaitre JF, Alix-Panabières C, Pujol P, Giraudeau M, Ujvari B, Thomas F. Dujon AM, et al. Proc Biol Sci. 2023 Jun 28;290(2001):20230940. doi: 10.1098/rspb.2023.0940. Epub 2023 Jun 28. Proc Biol Sci. 2023. PMID: 37357861 Free PMC article. - Human embryo implantation.
Muter J, Lynch VJ, McCoy RC, Brosens JJ. Muter J, et al. Development. 2023 May 15;150(10):dev201507. doi: 10.1242/dev.201507. Epub 2023 May 31. Development. 2023. PMID: 37254877 Free PMC article. Review.
References
- Archibald J. D., Rose K. D. In: The Rise of Placental Mammals. Rose K. D., Archibald J. D., editors. Baltimore: Johns Hopkins Univ. Press; 2005. pp. 1–8.
- Benirschke K., Kaufmann P. Pathology of the Human Placenta. New York: Springer; 2000.
- Enders A. C. Am. J. Anat. 1965;116:29–67. - PubMed
- Mossman H. W. Vertebrate Fetal Membranes: Comparative Ontogeny and Morphology, Evolution, Phylogenetic Significance, Basic Functions, Research Opportunities. New Brunswick, NJ: Rutgers Univ. Press; 1987.
- Kaufman P. Placenta Suppl. 1981;1:13–28.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources