PPARdelta regulates glucose metabolism and insulin sensitivity - PubMed (original) (raw)
PPARdelta regulates glucose metabolism and insulin sensitivity
Chih-Hao Lee et al. Proc Natl Acad Sci U S A. 2006.
Abstract
The metabolic syndrome is a collection of obesity-related disorders. The peroxisome proliferator-activated receptors (PPARs) regulate transcription in response to fatty acids and, as such, are potential therapeutic targets for these diseases. We show that PPARdelta (NR1C2) knockout mice are metabolically less active and glucose-intolerant, whereas receptor activation in db/db mice improves insulin sensitivity. Euglycemic-hyperinsulinemic-clamp experiments further demonstrate that a PPARdelta-specific agonist suppresses hepatic glucose output, increases glucose disposal, and inhibits free fatty acid release from adipocytes. Unexpectedly, gene array and functional analyses suggest that PPARdelta ameliorates hyperglycemia by increasing glucose flux through the pentose phosphate pathway and enhancing fatty acid synthesis. Coupling increased hepatic carbohydrate catabolism with its ability to promote beta-oxidation in muscle allows PPARdelta to regulate metabolic homeostasis and enhance insulin action by complementary effects in distinct tissues. The combined hepatic and peripheral actions of PPARdelta suggest new therapeutic approaches to treat type II diabetes.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
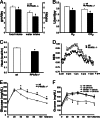
Fig. 1.
Depressed metabolism and glucose intolerance in PPARδ null mice. Metabolic parameters of wild-type and PPARδ null mice were measured in metabolic cages (n = 8). PPARδ null mice ate and drank less (A), consumed and produced less O2 and CO2 (B), expended less energy (presented as heat) (C), and have lower RERs (RER = VCO2/VO2) (D), particularly in the fed state (6 p.m. to 6 a.m.). (E) The glucose-tolerance test (GTT), showing that PPARδ null animals were glucose-intolerant on normal chow. (F) The GTT, showing a receptor-dependent effect of a PPARδ agonist (GW) on improving glucose tolerance. Both wild-type and PPARδ−/− mice were placed on a high-fat high-carbohydrate diet for 10 weeks, followed by a 2-week ligand treatment (n = 4). Statistical differences were observed only between vehicle- and ligand (GW)-treated wild-type mice. ∗, P < 0.05.
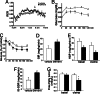
Fig. 2.
Treatment with a PPARδ agonist increases insulin sensitivity in db/db mice. PPARδ ligand treatment increased RER (A) and improved performance in the glucose-tolerance test (B) and insulin-tolerance test (C) in db/db mice (n = 10). In the insulin-tolerance test, glucose levels after insulin injection were presented as the percentage of initial glucose concentrations. (D_–_G) Euglycemic–hyperinsulinemic clamp in db/db mice, showing improved insulin sensitivity in multiple tissues after ligand treatment. PPARδ ligand-treated mice have a higher glucose infusion rate (GIR) (D), decreased hepatic glucose production (HGP) (F), increased insulin stimulated-glucose disposal rate (IS-GDR) (G), and lower free fatty acid levels after clamp (F). Vehicle (□); GW501516 (■); ∗, P < 0.05
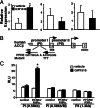
Fig. 3.
PPARδ regulates hepatic glucose and fatty acid homeostasis through direct transcriptional regulation. (A) Q-PCR analyses demonstrating increased ACCβ expression in livers of GW-treated db/db mice (Left). GW had no effects on levels of SREBP-1c (Center) or HMG-CoA reductase (Right). (B) The 5′ regulatory regions of the human _ACC_β gene. ACCβ is controlled by two promoters. A putative PPARδ response element (PPRE) was identified in the promoter I (−480 to −468, relative to the transcriptional starting site). Residues that were mutated in the reporter construct [PI (0.58 kb/M)] are indicated. (C) _ACC_β is a direct target gene of PPARδ. HepG2 cells were cotransfected with a luciferase reporter driven by the promoter I or II of the ACCβ gene, together with control vectors or expression vectors for PPARδ and the heterodimer partner RXRα, as well as a β-galactosidase internal control. Cells were treated with GW at 0.1 μM for 16 h. PPARδ increases the activity of the promoter I in a PPRE-dependent manner. PI (0.58 kb), the luciferase reporter driven by 0.58-kb 5′ upstream region of the promoter; I, PI (0.58 kb/M); 0.58-kb promoter I with PPRE mutations; PII (1.3 kb), 1.3-kb promoter II.
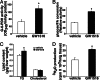
Fig. 4.
PPARδ promotes glucose flux to the pentose phosphate pathway and fatty acid synthesis in liver. (A) PPARδ ligand activates the pentose phosphate pathway. GW treatment increases the activity of glucose-6-phosphate dehydrogenase, the first enzyme in the pentose phosphate pathway, by 2-fold. The activities were determined by NADPH produced (OD 340 nm) by using liver lysates from vehicle or GW-treated db/db mice (n = 10). (B) PPARδ increases hepatic glucose-to-fatty acid conversion. Primary hepatocytes were isolated from vehicle or GW-treated db/db mice and loaded with [14C]glucose. The amount of glucose-derived fatty acid was determined by the radioactivity recovered from the organic phase after lipid extraction and was normalized to protein concentration. (C) Increased hepatic TG, but not cholesterol, content in ligand-treated db/db mice. (D) PPARδ increases β-oxidation in muscle. Soleus muscle strips were isolated from vehicle or GW-treated db/db mice and loaded with [3H]palmitic acid. The catabolic rate was determined by measuring the fatty acid β-oxidation product 3H2O and normalized to tissue weight. Vehicle (□); GW501516 (■); ∗, P < 0.05.
Similar articles
- PPARdelta is a fatty acid sensor that enhances mitochondrial oxidation in insulin-secreting cells and protects against fatty acid-induced dysfunction.
Ravnskjaer K, Frigerio F, Boergesen M, Nielsen T, Maechler P, Mandrup S. Ravnskjaer K, et al. J Lipid Res. 2010 Jun;51(6):1370-9. doi: 10.1194/jlr.M001123. Epub 2009 Nov 30. J Lipid Res. 2010. PMID: 19965574 Free PMC article. - Improved insulin sensitivity and islet function after PPARdelta activation in diabetic db/db mice.
Winzell MS, Wulff EM, Olsen GS, Sauerberg P, Gotfredsen CF, Ahrén B. Winzell MS, et al. Eur J Pharmacol. 2010 Jan 25;626(2-3):297-305. doi: 10.1016/j.ejphar.2009.09.053. Epub 2009 Oct 8. Eur J Pharmacol. 2010. PMID: 19818749 - CD36-dependent regulation of muscle FoxO1 and PDK4 in the PPAR delta/beta-mediated adaptation to metabolic stress.
Nahlé Z, Hsieh M, Pietka T, Coburn CT, Grimaldi PA, Zhang MQ, Das D, Abumrad NA. Nahlé Z, et al. J Biol Chem. 2008 May 23;283(21):14317-26. doi: 10.1074/jbc.M706478200. Epub 2008 Feb 28. J Biol Chem. 2008. PMID: 18308721 Free PMC article. - Novel approach to treat insulin resistance, type 2 diabetes, and the metabolic syndrome: simultaneous activation of PPARalpha, PPARgamma, and PPARdelta.
Evans JL, Lin JJ, Goldfine ID. Evans JL, et al. Curr Diabetes Rev. 2005 Aug;1(3):299-307. doi: 10.2174/157339905774574365. Curr Diabetes Rev. 2005. PMID: 18220606 Review. - Roles of peroxisome proliferator-activated receptor delta (PPARdelta) in the control of fatty acid catabolism. A new target for the treatment of metabolic syndrome.
Luquet S, Lopez-Soriano J, Holst D, Gaudel C, Jehl-Pietri C, Fredenrich A, Grimaldi PA. Luquet S, et al. Biochimie. 2004 Nov;86(11):833-7. doi: 10.1016/j.biochi.2004.09.024. Biochimie. 2004. PMID: 15589693 Review.
Cited by
- Emerging Roles of Dyslipidemia and Hyperglycemia in Diabetic Retinopathy: Molecular Mechanisms and Clinical Perspectives.
Rao H, Jalali JA, Johnston TP, Koulen P. Rao H, et al. Front Endocrinol (Lausanne). 2021 Mar 22;12:620045. doi: 10.3389/fendo.2021.620045. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33828528 Free PMC article. Review. - Molecular Characterization and Expression Analysis of the Peroxisome Proliferator Activated Receptor Delta (PPARδ) Gene before and after Exercise in Horse.
Cho HW, Shin S, Park JW, Choi JY, Kim NY, Lee WK, Lee HK, Song KD, Cho BW. Cho HW, et al. Asian-Australas J Anim Sci. 2015 May;28(5):697-702. doi: 10.5713/ajas.14.0575. Asian-Australas J Anim Sci. 2015. PMID: 25924962 Free PMC article. - Peroxisome proliferator-activated receptor (PPAR) α and δ activators induce ICAM-1 expression in quiescent non stimulated endothelial cells.
Naidenow J, Hrgovic I, Doll M, Hailemariam-Jahn T, Lang V, Kleemann J, Kippenberger S, Kaufmann R, Zöller N, Meissner M. Naidenow J, et al. J Inflamm (Lond). 2016 Aug 20;13:27. doi: 10.1186/s12950-016-0135-2. eCollection 2016. J Inflamm (Lond). 2016. PMID: 27547125 Free PMC article. - Telmisartan improves insulin resistance of skeletal muscle through peroxisome proliferator-activated receptor-δ activation.
Li L, Luo Z, Yu H, Feng X, Wang P, Chen J, Pu Y, Zhao Y, He H, Zhong J, Liu D, Zhu Z. Li L, et al. Diabetes. 2013 Mar;62(3):762-74. doi: 10.2337/db12-0570. Epub 2012 Dec 13. Diabetes. 2013. PMID: 23238297 Free PMC article. - ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1.
Haemmerle G, Moustafa T, Woelkart G, Büttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, Schreiber R, Eichmann T, Kolb D, Kotzbeck P, Schweiger M, Kumari M, Eder S, Schoiswohl G, Wongsiriroj N, Pollak NM, Radner FP, Preiss-Landl K, Kolbe T, Rülicke T, Pieske B, Trauner M, Lass A, Zimmermann R, Hoefler G, Cinti S, Kershaw EE, Schrauwen P, Madeo F, Mayer B, Zechner R. Haemmerle G, et al. Nat Med. 2011 Aug 21;17(9):1076-85. doi: 10.1038/nm.2439. Nat Med. 2011. PMID: 21857651 Free PMC article.
References
- Reaven G. M. J. Intern. Med. Suppl. 1994;736:13–22. - PubMed
- Chawla A., Repa J. J., Evans R. M., Mangelsdorf D. J. Science. 2001;294:1866–1870. - PubMed
- Lee C. H., Olson P., Evans R. M. Endocrinology. 2003;144:2201–2207. - PubMed
- Desvergne B., Wahli W. Endocr. Rev. 1999;20:649–688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19DK62434-01/DK/NIDDK NIH HHS/United States
- 5R37DK057978/DK/NIDDK NIH HHS/United States
- U19 DK062434/DK/NIDDK NIH HHS/United States
- 5P50HL56989/HL/NHLBI NIH HHS/United States
- P50 HL056989/HL/NHLBI NIH HHS/United States
- R37 DK057978/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous