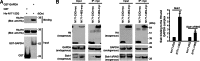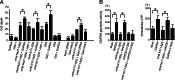Mutant huntingtin: nuclear translocation and cytotoxicity mediated by GAPDH - PubMed (original) (raw)
Mutant huntingtin: nuclear translocation and cytotoxicity mediated by GAPDH
Byoung-Il Bae et al. Proc Natl Acad Sci U S A. 2006.
Abstract
The pathophysiology of Huntington's disease reflects actions of mutant Huntingtin (Htt) (mHtt) protein with polyglutamine repeats, whose N-terminal fragment translocates to the nucleus to elicit neurotoxicity. We establish that the nuclear translocation and associated cytotoxicity of mHtt reflect a ternary complex of mHtt with GAPDH and Siah1, a ubiquitin-E3-ligase. Overexpression of GAPDH or Siah1 enhances nuclear translocation of mHtt and cytotoxicity, whereas GAPDH mutants that cannot bind Siah1 prevent translocation. Depletion of GAPDH or Siah1 by RNA interference diminishes nuclear translocation of mHtt.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
Htt, GAPDH, and Siah1 form a ternary complex. (A) GAPDH binds to Htt in vitro. GST-tagged GAPDH (GST-GAPDH) and His-tagged Htt containing N-terminal 171 amino acids and 23 polyQ (His-N171-23Q) were purified from Escherichia coli. In vitro binding assay was performed on glutathione beads. (B) Htt, GAPDH, and Siah1 form a ternary complex in HEK293T cells transfected with myc-tagged Htt N171-23Q or N171-148Q, and HA-tagged Siah1 or Siah1ΔRING, as shown by coimmunoprecipitation (co-IP). The Htt protein complex was immunoprecipitated by myc antibody and immunoblotted with Htt (EM48), GAPDH, and HA antibodies. (Left) Siah1, a rapidly turning-over protein, is stabilized (input) and recruited to the Htt-GAPDH complex (IP) to a greater extent with N171-148Q than with N171-23Q. (Center) Siah1ΔRING is resistant to self degradation, leading to comparable protein levels in N171-23Q and N171-148Q-transfected cells (input). More Siah1ΔRING is recruited to the Htt-GAPDH complex by N171-148Q than by N171-23Q (IP). PolyQ expansion does not affect the interaction of GAPDH and Htt. (Right) Densitometric analysis of coIP shows that mHtt is better than wtHtt in recruiting Siah1 and Siah1ΔRING. Data represent the mean and SEM of two independent experiments (t test, ∗, P < 0.01).
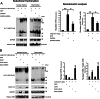
Fig. 2.
Overexpressed GAPDH and Siah1 enhance nuclear targeting of mHtt. (A) Overexpressed GAPDH augments nuclear targeting of mHtt (N171-82Q) 10- to 15-fold in N2a cells transfected with FLAG-tagged mHtt and GAPDH. The binding of GAPDH to Siah1 is critical for nuclear targeting of mHtt, because GAPDH-K225A, which fails in binding to Siah1 and translocating to the nucleus, leads to significantly less nuclear mHtt than GAPDH (t test, ∗, P < 0.01; ∗∗, P < 0.001). (B) Overexpressed Siah1 augments nuclear targeting of mHtt in N2a cells transfected with mHtt and Siah1. Overexpressed Siah1 and Siah1ΔNLS degrade both total and nuclear mHtt through the ubiquitin-E3-ligase activity of Siah1, as Siah1 lacking ubiquitin-E3-ligase activity (Siah1ΔRING) only increases nuclear targeting of mHtt 2- to 3-fold without degrading mHtt. It is noticeable that the ratio of nuclear to total mHtt is tripled by overexpression of Siah1, but not Siah1ΔNLS (ANOVA, ∗, P < 0.01). The bracket indicates SDS-insoluble mHtt aggregates. Data represent the mean and SEM of three independent experiments.
Fig. 3.
Overexpressed GAPDH and Siah1ΔRING increase mHtt cytotoxicity. (A) Overexpressed GAPDH and Siah1ΔRING augment mHtt cytotoxicity in N2a cells transfected with Htt and GAPDH, Htt-Associated Protein-1 (HAP-1), Siah1ΔRING, or Siah1ΔNLS for 72 h. GAPDH, but not HAP-1, augments cytotoxicity of mHtt N171-82Q, mHtt N427-148Q (mHtt containing N-terminal 427 amino acids and 148 polyQ), and 82 polyQ (82Q), suggesting the specificity of GAPDH. GAPDH is not toxic in itself or with 23 polyQ (23Q). Siah1ΔRING, but not Siah1ΔNLS, significantly augments mHtt toxicity. (B) Enhanced mHtt cytotoxicity by GAPDH is independent of GAPDH glycolytic activity or intracellular ATP levels. Overexpressed GAPDH augments glycolytic activity and the ATP level, which is not affected by mHtt N171-82Q expression. GAPDH-C150S harbors a mutation at the catalytic center, debilitating enzymatic activity of GAPDH (t test, ∗, P < 0.01).
Fig. 4.
Depletion of GAPDH and Siah1 blocks nuclear targeting of mHtt. mHtt containing N-terminal 67 amino acids and 104Q fused to GFP (N67-104Q-GFP) was transfected into N2a cells pretreated with siRNA to GAPDH or Siah1. Medium was supplemented with 1 mM pyruvate to avoid toxicity of GAPDH siRNA. Intranuclear inclusions are frequently observed with control RNAi but not with GAPDH siRNA or Siah1 siRNA. When nuclear targeting of mHtt is significantly less efficient without GAPDH or Siah1, and mHtt is accumulated in the perinuclear region. Each graph bar in B corresponds to the scoring of ≈200 inclusions from randomly chosen fields 36 h after transfection performed in triplicate (t test, ∗, P < 0.01).
Similar articles
- S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding.
Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, Ferris CD, Hayward SD, Snyder SH, Sawa A. Hara MR, et al. Nat Cell Biol. 2005 Jul;7(7):665-74. doi: 10.1038/ncb1268. Epub 2005 Jun 12. Nat Cell Biol. 2005. PMID: 15951807 - Overexpression and nuclear accumulation of glyceraldehyde-3-phosphate dehydrogenase in a transgenic mouse model of Huntington's disease.
Senatorov VV, Charles V, Reddy PH, Tagle DA, Chuang DM. Senatorov VV, et al. Mol Cell Neurosci. 2003 Mar;22(3):285-97. doi: 10.1016/s1044-7431(02)00013-1. Mol Cell Neurosci. 2003. PMID: 12691731 - Huntingtin is cleaved by caspases in the cytoplasm and translocated to the nucleus via perinuclear sites in Huntington's disease patient lymphoblasts.
Sawa A, Nagata E, Sutcliffe S, Dulloor P, Cascio MB, Ozeki Y, Roy S, Ross CA, Snyder SH. Sawa A, et al. Neurobiol Dis. 2005 Nov;20(2):267-74. doi: 10.1016/j.nbd.2005.02.013. Neurobiol Dis. 2005. PMID: 15890517 - Nitric oxide-GAPDH-Siah: a novel cell death cascade.
Hara MR, Snyder SH. Hara MR, et al. Cell Mol Neurobiol. 2006 Jul-Aug;26(4-6):527-38. doi: 10.1007/s10571-006-9011-6. Epub 2006 Apr 22. Cell Mol Neurobiol. 2006. PMID: 16633896 Review. - GAPDH as a sensor of NO stress.
Hara MR, Cascio MB, Sawa A. Hara MR, et al. Biochim Biophys Acta. 2006 May;1762(5):502-9. doi: 10.1016/j.bbadis.2006.01.012. Epub 2006 Mar 9. Biochim Biophys Acta. 2006. PMID: 16574384 Review.
Cited by
- Balancing reactivity against selectivity: the evolution of protein S-nitrosylation as an effector of cell signaling by nitric oxide.
Derakhshan B, Hao G, Gross SS. Derakhshan B, et al. Cardiovasc Res. 2007 Jul 15;75(2):210-9. doi: 10.1016/j.cardiores.2007.04.023. Epub 2007 May 3. Cardiovasc Res. 2007. PMID: 17524376 Free PMC article. Review. - Functional consequences of piceatannol binding to glyceraldehyde-3-phosphate dehydrogenase.
Gerszon J, Serafin E, Buczkowski A, Michlewska S, Bielnicki JA, Rodacka A. Gerszon J, et al. PLoS One. 2018 Jan 3;13(1):e0190656. doi: 10.1371/journal.pone.0190656. eCollection 2018. PLoS One. 2018. PMID: 29298351 Free PMC article. - Homocysteine induces glyceraldehyde-3-phosphate dehydrogenase acetylation and apoptosis in the neuroblastoma cell line Neuro2a.
Fang M, Jin A, Zhao Y, Liu X. Fang M, et al. Braz J Med Biol Res. 2016 Feb;49(2):e4543. doi: 10.1590/1414-431X20154543. Epub 2016 Jan 19. Braz J Med Biol Res. 2016. PMID: 26785692 Free PMC article. - Antioxidants in Huntington's disease.
Johri A, Beal MF. Johri A, et al. Biochim Biophys Acta. 2012 May;1822(5):664-74. doi: 10.1016/j.bbadis.2011.11.014. Epub 2011 Nov 23. Biochim Biophys Acta. 2012. PMID: 22138129 Free PMC article. Review. - The Novel Alpha-2 Adrenoceptor Inhibitor Beditin Reduces Cytotoxicity and Huntingtin Aggregates in Cell Models of Huntington's Disease.
Singer E, Hunanyan L, Melkonyan MM, Weber JJ, Danielyan L, Nguyen HP. Singer E, et al. Pharmaceuticals (Basel). 2021 Mar 12;14(3):257. doi: 10.3390/ph14030257. Pharmaceuticals (Basel). 2021. PMID: 33809220 Free PMC article.
References
- The Huntington’s Disease Collaborative Research Group. Cell. 1993;72:971–983. - PubMed
- Peters M. F., Nucifora F. C., Jr., Kushi J., Seaman H. C., Cooper J. K., Herring W. J., Dawson V. L., Dawson T. M., Ross C. A. Mol. Cell. Neurosci. 1999;14:121–128. - PubMed
- Saudou F., Finkbeiner S., Devys D., Greenberg M. E. Cell. 1998;95:55–66. - PubMed
- Hodgson J. G., Agopyan N., Gutekunst C. A., Leavitt B. R., LePiane F., Singaraja R., Smith D. J., Bissada N., McCutcheon K., Nasir J., et al. Neuron. 1999;23:181–192. - PubMed
- Wheeler V. C., White J. K., Gutekunst C. A., Vrbanac V., Weaver M., Li X. J., Li S. H., Yi H., Vonsattel J. P., Gusella J. F., et al. Hum. Mol. Genet. 2000;9:503–513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH-069853/MH/NIMH NIH HHS/United States
- DA-00266/DA/NIDA NIH HHS/United States
- R01 MH069853/MH/NIMH NIH HHS/United States
- DA-00074/DA/NIDA NIH HHS/United States
- P50 DA000266/DA/NIDA NIH HHS/United States
- K05 DA000074/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials